Complex Arrhythmia Syndrome in a Knock-In Mouse Model Carrier of the N98S Calm1 Mutation
- PMID: 32929985
- PMCID: PMC7867118
- DOI: 10.1161/CIRCULATIONAHA.120.046450
Complex Arrhythmia Syndrome in a Knock-In Mouse Model Carrier of the N98S Calm1 Mutation
Abstract
Background: Calmodulin mutations are associated with arrhythmia syndromes in humans. Exome sequencing previously identified a de novo mutation in CALM1 resulting in a p.N98S substitution in a patient with sinus bradycardia and stress-induced bidirectional ventricular ectopy. The objectives of the present study were to determine if mice carrying the N98S mutation knocked into Calm1 replicate the human arrhythmia phenotype and to examine arrhythmia mechanisms.
Methods: Mouse lines heterozygous for the Calm1N98S allele (Calm1N98S/+) were generated using CRISPR/Cas9 technology. Adult mutant mice and their wildtype littermates (Calm1+/+) underwent electrocardiographic monitoring. Ventricular de- and repolarization was assessed in isolated hearts using optical voltage mapping. Action potentials and whole-cell currents and [Ca2+]i, as well, were measured in single ventricular myocytes using the patch-clamp technique and fluorescence microscopy, respectively. The microelectrode technique was used for in situ membrane voltage monitoring of ventricular conduction fibers.
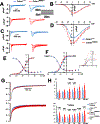
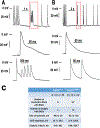
Results: Two biologically independent knock-in mouse lines heterozygous for the Calm1N98S allele were generated. Calm1N98S/+ mice of either sex and line exhibited sinus bradycardia, QTc interval prolongation, and catecholaminergic bidirectional ventricular tachycardia. Male mutant mice also showed QRS widening. Pharmacological blockade and activation of β-adrenergic receptors rescued and exacerbated, respectively, the long-QT phenotype of Calm1N98S/+ mice. Optical and electric assessment of membrane potential in isolated hearts and single left ventricular myocytes, respectively, revealed β-adrenergically induced delay of repolarization. β-Adrenergic stimulation increased peak density, slowed inactivation, and left-shifted the activation curve of ICa.L significantly more in Calm1N98S/+ versus Calm1+/+ ventricular myocytes, increasing late ICa.L in the former. Rapidly paced Calm1N98S/+ ventricular myocytes showed increased propensity to delayed afterdepolarization-induced triggered activity, whereas in situ His-Purkinje fibers exhibited increased susceptibility for pause-dependent early afterdepolarizations. Epicardial mapping of Calm1N98S/+ hearts showed that both reentry and focal mechanisms contribute to arrhythmogenesis.
Conclusions: Heterozygosity for the Calm1N98S mutation is causative of an arrhythmia syndrome characterized by sinus bradycardia, QRS widening, adrenergically mediated QTc interval prolongation, and bidirectional ventricular tachycardia. β-Adrenergically induced ICa.L dysregulation contributes to the long-QT phenotype. Pause-dependent early afterdepolarizations and tachycardia-induced delayed afterdepolarizations originating in the His-Purkinje network and ventricular myocytes, respectively, constitute potential sources of arrhythmia in Calm1N98S/+ hearts.
Keywords: arrhythmias, cardiac; calcium channels; calmodulin; heart diseases; model, animal.
Figures






Similar articles
-
Antisense Oligonucleotide Therapy for Calmodulinopathy.Circulation. 2024 Oct 8;150(15):1199-1210. doi: 10.1161/CIRCULATIONAHA.123.068111. Epub 2024 Aug 19. Circulation. 2024. PMID: 39155863
-
K+ currents in ventricular cardiomyocytes of p.N98S-calmodulin mutant mice.Am J Physiol Heart Circ Physiol. 2025 Mar 1;328(3):H658-H675. doi: 10.1152/ajpheart.00470.2024. Epub 2024 Dec 31. Am J Physiol Heart Circ Physiol. 2025. PMID: 39739562
-
Sex and Gene Influence Arrhythmia Susceptibility in Murine Models of Calmodulinopathy.Circ Arrhythm Electrophysiol. 2023 Sep;16(9):e010891. doi: 10.1161/CIRCEP.122.010891. Epub 2023 Aug 17. Circ Arrhythm Electrophysiol. 2023. PMID: 37589122 Free PMC article.
-
Genetic Loss of IK1 Causes Adrenergic-Induced Phase 3 Early Afterdepolariz ations and Polymorphic and Bidirectional Ventricular Tachycardia.Circ Arrhythm Electrophysiol. 2020 Sep;13(9):e008638. doi: 10.1161/CIRCEP.120.008638. Epub 2020 Aug 4. Circ Arrhythm Electrophysiol. 2020. PMID: 32931337 Free PMC article.
-
Allele-specific ablation rescues electrophysiological abnormalities in a human iPS cell model of long-QT syndrome with a CALM2 mutation.Hum Mol Genet. 2017 May 1;26(9):1670-1677. doi: 10.1093/hmg/ddx073. Hum Mol Genet. 2017. PMID: 28335032
Cited by
-
Antisense Oligonucleotide Therapy for Calmodulinopathy.Circulation. 2024 Oct 8;150(15):1199-1210. doi: 10.1161/CIRCULATIONAHA.123.068111. Epub 2024 Aug 19. Circulation. 2024. PMID: 39155863
-
Advancements in the diagnosis and management of premature ventricular contractions in pediatric patients.Front Pediatr. 2024 Mar 20;12:1373772. doi: 10.3389/fped.2024.1373772. eCollection 2024. Front Pediatr. 2024. PMID: 38571703 Free PMC article. Review.
-
Human calmodulin mutations cause arrhythmia and affect neuronal function in C. elegans.Hum Mol Genet. 2023 Jun 5;32(12):2068-2083. doi: 10.1093/hmg/ddad042. Hum Mol Genet. 2023. PMID: 36920509 Free PMC article.
-
Understanding Calmodulin Variants Affecting Calcium-Dependent Inactivation of L-Type Calcium Channels through Whole-Cell Simulation of the Cardiac Ventricular Myocyte.Biomolecules. 2022 Dec 29;13(1):72. doi: 10.3390/biom13010072. Biomolecules. 2022. PMID: 36671457 Free PMC article.
-
NaV1.6 dysregulation within myocardial T-tubules by D96V calmodulin enhances proarrhythmic sodium and calcium mishandling.J Clin Invest. 2023 Apr 3;133(7):e152071. doi: 10.1172/JCI152071. J Clin Invest. 2023. PMID: 36821382 Free PMC article.
References
-
- Boczek NJ, Gomez-Hurtado N, Ye D, Calvert ML, Tester DJ, Kryshtal D, Hwang HS, Johnson CN, Chazin WJ, Loporcaro CG, et al. Spectrum and Prevalence of CALM1-, CALM2-, and CALM3-Encoded Calmodulin Variants in Long QT Syndrome and Functional Characterization of a Novel Long QT Syndrome-Associated Calmodulin Missense Variant, E141G. Circ Cardiovasc Genet. 2016;9:136–146. - PMC - PubMed
-
- Gomez-Hurtado N, Boczek NJ, Kryshtal DO, Johnson CN, Sun J, Nitu FR, Cornea RL, Chazin WJ, Calvert ML, Tester DJ, et al. Novel CPVT-Associated Calmodulin Mutation in CALM3 (CALM3-A103V) Activates Arrhythmogenic Ca2+ Waves and Sparks. Circ Arrhythm Electrophysiol. 2016;9: pii: e004161. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

