Impaired State-Dependent Potentiation of GABAergic Synaptic Currents Triggers Seizures in a Genetic Generalized Epilepsy Model
- PMID: 32930324
- PMCID: PMC7906787
- DOI: 10.1093/cercor/bhaa256
Impaired State-Dependent Potentiation of GABAergic Synaptic Currents Triggers Seizures in a Genetic Generalized Epilepsy Model
Abstract
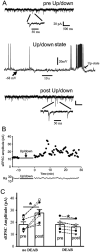
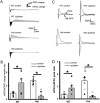
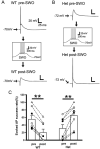
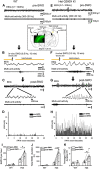
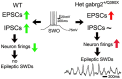
Epileptic activity in genetic generalized epilepsy (GGE) patients preferentially appears during sleep and its mechanism remains unknown. Here, we found that sleep-like slow-wave oscillations (0.5 Hz SWOs) potentiated excitatory and inhibitory synaptic currents in layer V cortical pyramidal neurons from wild-type (wt) mouse brain slices. In contrast, SWOs potentiated excitatory, but not inhibitory, currents in cortical neurons from a heterozygous (het) knock-in (KI) Gabrg2+Q/390X model of Dravet epilepsy syndrome. This created an imbalance between evoked excitatory and inhibitory currents to effectively prompt neuronal action potential firings. Similarly, physiologically similar up-/down-state induction (present during slow-wave sleep) in cortical neurons also potentiated excitatory synaptic currents within brain slices from wt and het KI mice. Moreover, this state-dependent potentiation of excitatory synaptic currents entailed some signaling pathways of homeostatic synaptic plasticity. Consequently, in het KI mice, in vivo SWO induction (using optogenetic methods) triggered generalized epileptic spike-wave discharges (SWDs), being accompanied by sudden immobility, facial myoclonus, and vibrissa twitching. In contrast, in wt littermates, SWO induction did not cause epileptic SWDs and motor behaviors. To our knowledge, this is the first mechanism to explain why epileptic SWDs preferentially happen during non rapid eye-movement sleep and quiet-wakefulness in human GGE patients.
Keywords: genetic generalized epilepsy; homeostatic synaptic plasticity; inhibitory synaptic currents; seizure onset; slow-wave oscillation.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures



Comment in
-
You Snooze You Seize: GABAergic Potentiation of Genetic Generalized Seizures During NREM.Epilepsy Curr. 2021 May 6;21(4):290-292. doi: 10.1177/15357597211012454. eCollection 2021 Jul-Aug. Epilepsy Curr. 2021. PMID: 34690570 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

