Optimizing Pharmacology Studies in Pregnant and Lactating Women Using Lessons From HIV: A Consensus Statement
- PMID: 32930408
- PMCID: PMC8167886
- DOI: 10.1002/cpt.2048
Optimizing Pharmacology Studies in Pregnant and Lactating Women Using Lessons From HIV: A Consensus Statement
Abstract
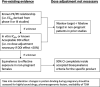
Information on the extent of drug exposure to mothers and infants during pregnancy and lactation normally becomes available years after regulatory approval of a drug. Clinicians face knowledge gaps on drug selection and dosing in pregnancy and infant exposure during breastfeeding. Physiological changes during pregnancy often result in lower drug exposures of antiretrovirals, and in some cases a risk of reduced virologic efficacy. The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network and the World Health Organization (WHO)-convened Pediatric Antiretrovirals Working Group collaboratively organized a workshop of key stakeholders in June 2019 to define key standards to generate pharmacology data for antiretrovirals to be used among pregnant and lactating women; review the antiretroviral product pipeline; describe key gaps for use in low-income and middle-income countries; and identify opportunities to undertake optimal studies allowing for rapid implementation in the clinical field. We discussed ethical and regulatory principles, systemic approaches to obtaining data for pregnancy pharmacokinetic/pharmacodynamic (PK/PD) studies, control groups, optimal sampling times during pregnancy, and pharmacokinetic parameters to be considered as primary end points in pregnancy PK/PD studies. For lactation studies, the type of milk to collect, ascertainment of maternal adherence, and optimal PK methods to estimate exposure were discussed. Participants strongly recommended completion of preclinical reproductive toxicology studies prior to phase III, to allow study protocols to include pregnant women or to allow women who become pregnant after enrolment to continue in the trial. The meeting concluded by developing an algorithm for design and interpretation of results and noted that recruitment of pregnant and lactating women into clinical trials is critical.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.C.: Research grant support from ViiV Healthcare, Gilead Sciences, Janssen Research, and Merck, paid to the institution. M.M.: Research grant support from ViiV Healthcare, Gilead Sciences, and Merck & Co, paid to the institution. J.M.: Research grant support from Gilead Sciences and Veloxis Pharmaceuticals. J.J.K.: Research support (paid to institution) from Gilead Sciences and ViiV Healthcare. All other authors declared no competing interests for this work.
Figures


References
-
- Tasnif, Y., Morado, J. & Hebert, M.F. Pregnancy‐related pharmacokinetic changes. Clin. Pharmacol. Ther. 100, 53–62 (2016). - PubMed
-
- Ke, A.B., Rostami‐Hodjegan, A., Zhao, P. & Unadkat, J.D. Pharmacometrics in pregnancy: an unmet need. Annu. Rev. Pharmacol. Toxicol. 54, 53–69 (2014). - PubMed
-
- Frederiksen, M.C.Physiologic changes in pregnancy and their effect on drug disposition. Semin. Perinatol. 25, 120–123 (2001). - PubMed
-
- Tracy, T.S., Venkataramanan, R., Glover, D.D. & Caritis, S.N. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am. J. Obstet. Gynecol. 192, 633–639 (2005). - PubMed

