Increasing the Complexity in the MIL-53 Structure: The Combination of the Mixed-Metal and the Mixed-Linker Concepts
- PMID: 32930421
- PMCID: PMC7898851
- DOI: 10.1002/chem.202003304
Increasing the Complexity in the MIL-53 Structure: The Combination of the Mixed-Metal and the Mixed-Linker Concepts
Abstract
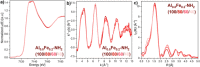
The isoreticular mixed-component concept is a promising approach to tailor the material properties of metal-organic frameworks. While isoreticular mixed-metal or mixed-linker materials are commonly synthesized, the combination of both concepts for the development of isoreticular materials featuring both two metals and two linkers is still rarely investigated. Herein, we present the development of mixed-metal/mixed-linker MIL-53 materials that contain different metal combinations (Al/Sc, Al/V, Al/Cr, Al/Fe) and different linker ratios (terephthalate/2-aminoterephthalate). The possibility of changing the metal combination and the linker ratio independently from each other enables a large variety of modifications. A thorough characterization (PXRD, ATR-IR, TGA, 1 H NMR, ICP-OES) confirmed that all components were incorporated into the framework structure with a statistical distribution. Nitrogen physisorption measurements showed that the breathing behavior can be tailored by adjusting the linker ratio for all metal combinations. All materials were successfully used for post-synthetic modification reactions with maleic anhydride.
Keywords: breathing behavior; isoreticular; metal-organic frameworks; mixed-metal/mixed-linker; physisorption.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interests.
Figures











References
-
- None
-
- Qin J. S., Yuan S., Wang Q., Alsalme A., Zhou H. C., J. Mater. Chem. A 2017, 5, 4280–4291;
-
- Li B., Wen H.-M., Cui Y., Zhou W., Qian G., Chen B., Adv. Mater. 2016, 28, 8819–8860; - PubMed
-
- Li B. Y., Chrzanowski M., Zhang Y. M., Ma S. Q., Coord. Chem. Rev. 2016, 307, 106–129.
-
- None
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

