Cross-Sectional Area Measurement Techniques of Soft Tissue: A Literature Review
- PMID: 32930465
- PMCID: PMC7767688
- DOI: 10.1111/os.12757
Cross-Sectional Area Measurement Techniques of Soft Tissue: A Literature Review
Abstract
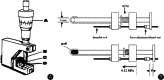
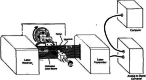
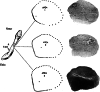
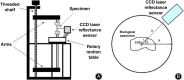
Evaluation of the biomechanical properties of soft tissues by measuring the stress-strain relationships has been the focus of numerous investigations. The accuracy of stress depends, in part, upon the determination of the cross-sectional area (CSA). However, the complex geometry and pliability of soft tissues, especially ligaments and tendons, make it difficult to obtain accurate CSA, and the development of CSA measurement methods of soft tissues continues. Early attempts to determine the CSA of soft tissues include gravimetric method, geometric approximation technique, area micrometer method, and microtomy technique. Since 1990, a series of new methods have emerged, including medical imaging techniques (e.g. magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound imaging (USI)), laser techniques (e.g. the laser micrometer method, the linear laser scanner (LLS) technique, and the laser reflection system (LRS) method), molding techniques, and three-dimensional (3D) scanning techniques.
Keywords: Area measurement; Biomechanics; Cross-sectional area; Soft tissues.
© 2020 The Authors. Orthopaedic Surgery published by Chinese Orthopaedic Association and John Wiley & Sons Australia, Ltd.
Figures










References
-
- Elden HR. Aging of rat tail tendons. J Gerontol, 1964, 19: 173–178. - PubMed
-
- Abrahams M. Mechanical behaviour of tendon in vitro: a preliminary report. Med Biol Eng, 1967, 5: 433–443. - PubMed
-
- Matthews LS, Ellis D. Viscoelastic properties of cat tendon: effects of time after death and preservation by freezing. J Biomech, 1968, 1: 65–71. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

