Aryloxy Diester Phosphonamidate Prodrugs of Phosphoantigens (ProPAgens) as Potent Activators of Vγ9/Vδ2 T-Cell Immune Responses
- PMID: 32930595
- PMCID: PMC7549095
- DOI: 10.1021/acs.jmedchem.0c01232
Aryloxy Diester Phosphonamidate Prodrugs of Phosphoantigens (ProPAgens) as Potent Activators of Vγ9/Vδ2 T-Cell Immune Responses
Abstract
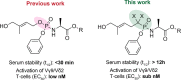
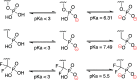
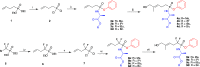
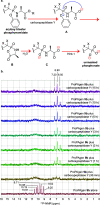
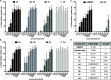
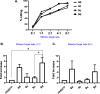
Vγ9/Vδ2 T-cells are activated by pyrophosphate-containing small molecules known as phosphoantigens (PAgs). The presence of the pyrophosphate group in these PAgs has limited their drug-like properties because of its instability and polar nature. In this work, we report a novel and short Grubbs olefin metathesis-mediated synthesis of methylene and difluoromethylene monophosphonate derivatives of the PAg (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBP) as well as their aryloxy diester phosphonamidate prodrugs, termed ProPAgens. These prodrugs showed excellent stability in human serum (t1/2 > 12 h) and potent activation of Vγ9/Vδ2 T-cells (EC50 ranging from 5 fM to 73 nM), which translated into sub-nanomolar γδ T-cell-mediated eradication of bladder cancer cells in vitro. Additionally, a combination of in silico and in vitro enzymatic assays demonstrated the metabolism of these phosphonamidates to release the unmasked PAg monophosphonate species. Collectively, this work establishes HMBP monophosphonate ProPAgens as ideal candidates for further investigation as novel cancer immunotherapeutic agents.
Conflict of interest statement
The authors declare the following competing financial interest(s): Y.M. and B.E.W. are named inventors on a patent application filed by Cardiff University (GB1810965.2), which covers the compounds discussed in this work.
Figures
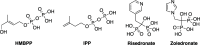
Similar articles
-
Phosphonodiamidate prodrugs of phosphoantigens (ProPAgens) exhibit potent Vγ9/Vδ2 T cell activation and eradication of cancer cells.RSC Med Chem. 2024 Jun 3;15(7):2462-2473. doi: 10.1039/d4md00208c. eCollection 2024 Jul 17. RSC Med Chem. 2024. PMID: 39026632 Free PMC article.
-
Phosphonamidate Prodrugs of a Butyrophilin Ligand Display Plasma Stability and Potent Vγ9 Vδ2 T Cell Stimulation.J Med Chem. 2018 Oct 11;61(19):8658-8669. doi: 10.1021/acs.jmedchem.8b00655. Epub 2018 Sep 26. J Med Chem. 2018. PMID: 30199251 Free PMC article.
-
Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin.J Immunol. 2015 Mar 1;194(5):2390-8. doi: 10.4049/jimmunol.1401064. Epub 2015 Jan 30. J Immunol. 2015. PMID: 25637025 Free PMC article.
-
Non-peptide antigens activating human Vgamma9/Vdelta2 T lymphocytes.Immunol Lett. 2004 Sep;95(2):129-38. doi: 10.1016/j.imlet.2004.06.013. Immunol Lett. 2004. PMID: 15388252 Review.
-
γδ T-APCs: a novel tool for immunotherapy?Cell Mol Life Sci. 2011 Jul;68(14):2443-52. doi: 10.1007/s00018-011-0706-6. Epub 2011 May 15. Cell Mol Life Sci. 2011. PMID: 21573785 Free PMC article. Review.
Cited by
-
A Brief Molecular History of Vγ9Vδ2 TCR-Mediated Phosphoantigen Sensing.Immunol Rev. 2025 May;331(1):e70023. doi: 10.1111/imr.70023. Immunol Rev. 2025. PMID: 40181561 Free PMC article. Review.
-
Generation of Stable Isopentenyl Monophosphate Aryloxy Triester Phosphoramidates as Activators of Vγ9Vδ2 T Cells.ChemMedChem. 2021 Aug 5;16(15):2375-2380. doi: 10.1002/cmdc.202100198. Epub 2021 May 19. ChemMedChem. 2021. PMID: 33899332 Free PMC article.
-
Synthesis of Mixed Phosphonate Esters and Amino Acid-Based Phosphonamidates, and Their Screening as Herbicides.Int J Mol Sci. 2024 Apr 26;25(9):4739. doi: 10.3390/ijms25094739. Int J Mol Sci. 2024. PMID: 38731958 Free PMC article.
-
The Multifaceted MEP Pathway: Towards New Therapeutic Perspectives.Molecules. 2023 Feb 1;28(3):1403. doi: 10.3390/molecules28031403. Molecules. 2023. PMID: 36771066 Free PMC article. Review.
-
Synthesis and evaluation of triazole-containing aryl/acyloxy prodrugs of a BTN3A1 ligand.Eur J Med Chem. 2025 Apr 5;287:117345. doi: 10.1016/j.ejmech.2025.117345. Epub 2025 Feb 1. Eur J Med Chem. 2025. PMID: 39919440
References
-
- Morita C. T.; Jin C.; Sarikonda G.; Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007, 215, 59–76. 10.1111/j.1600-065x.2006.00479.x. - DOI - PubMed
-
- Shen Y.; Zhou D.; Qiu L.; Lai X.; Simon M.; Shen L.; Kou Z.; Wang Q.; Jiang L.; Estep J.; Hunt R.; Clagett M.; Sehgal P. K.; Li Y.; Zeng X.; Morita C. T.; Brenner M. B.; Letvin N. L.; Chen Z. W. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science 2002, 295, 2255–2258. 10.1126/science.1068819. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

