Sample preparation of bone tissue for MALDI-MSI for forensic and (pre)clinical applications
- PMID: 32930817
- PMCID: PMC8007508
- DOI: 10.1007/s00216-020-02920-1
Sample preparation of bone tissue for MALDI-MSI for forensic and (pre)clinical applications
Abstract
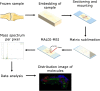
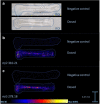
In the past decades, matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) has been applied to a broad range of biological samples, e.g., forensics and preclinical samples. The use of MALDI-MSI for the analysis of bone tissue has been limited due to the insulating properties of the material but more importantly the absence of a proper sample preparation protocol for undecalcified bone tissue. Undecalcified sections are preferred to retain sample integrity as much as possible or to study the tissue-bone bio interface in particular. Here, we optimized the sample preparation protocol of undecalcified bone samples, aimed at both targeted and untargeted applications for forensic and preclinical applications, respectively. Different concentrations of gelatin and carboxymethyl cellulose (CMC) were tested as embedding materials. The composition of 20% gelatin and 7.5% CMC showed to support the tissue best while sectioning. Bone tissue has to be sectioned with a tungsten carbide knife in a longitudinal fashion, while the sections need to be supported with double-sided tapes to maintain the morphology of the tissue. The developed sectioning method was shown to be applicable on rat and mouse as well as human bone samples. Targeted (methadone and EDDP) as well as untargeted (unknown lipids) detection was demonstrated. DHB proved to be the most suitable matrix for the detection of methadone and EDDP in positive ion mode. The limit of detection (LOD) is estimated to approximately 50 pg/spot on bone tissue. The protocol was successfully applied to detect the presence of methadone and EDDP in a dosed rat femur and a dosed human clavicle. The best matrices for the untargeted detection of unknown lipids in mouse hind legs in positive ion mode were CHCA and DHB based on the number of tissue-specific peaks and signal-to-noise ratios. The developed and optimized sample preparation method, applicable on animal and human bones, opens the door for future forensic and (pre)clinical investigations.
Keywords: Bone tissue; MALDI; Mass spectrometry imaging; Sample preparation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Preparation of multicellular spheroid sections embedded with sodium carboxymethyl cellulose for mass spectrometry imaging.Anal Methods. 2025 May 1;17(17):3407-3414. doi: 10.1039/d5ay00022j. Anal Methods. 2025. PMID: 40211921
-
Poly-l-lysine-based tissue embedding compatible with matrix-assisted laser desorption ionization-mass spectrometry imaging analysis of dry and fragile aristolochia plants.J Chromatogr A. 2019 Dec 20;1608:460389. doi: 10.1016/j.chroma.2019.460389. Epub 2019 Jul 29. J Chromatogr A. 2019. PMID: 31378528
-
Combined matrix-assisted laser desorption/ionisation-mass spectrometry imaging with liquid chromatography-tandem mass spectrometry for observing spatial distribution of lipids in whole Caenorhabditis elegans.Rapid Commun Mass Spectrom. 2024 Sep;38(17):e9850. doi: 10.1002/rcm.9850. Rapid Commun Mass Spectrom. 2024. PMID: 39034751
-
Surface-assisted laser desorption ionization mass spectrometry techniques for application in forensics.Mass Spectrom Rev. 2015 Nov-Dec;34(6):627-40. doi: 10.1002/mas.21431. Epub 2014 Jun 11. Mass Spectrom Rev. 2015. PMID: 24916100 Review.
-
Applications of MALDI mass spectrometry in forensic science.Anal Bioanal Chem. 2024 Oct;416(24):5255-5280. doi: 10.1007/s00216-024-05470-y. Epub 2024 Aug 20. Anal Bioanal Chem. 2024. PMID: 39160439 Review.
Cited by
-
In situ lipidomics of Staphylococcus aureus osteomyelitis using imaging mass spectrometry.bioRxiv [Preprint]. 2023 Dec 2:2023.12.01.569690. doi: 10.1101/2023.12.01.569690. bioRxiv. 2023. Update in: Cell Chem Biol. 2024 Oct 17;31(10):1852-1868.e5. doi: 10.1016/j.chembiol.2024.09.005. PMID: 38077019 Free PMC article. Updated. Preprint.
-
Sample Preparation Method for MALDI Mass Spectrometry Imaging of Fresh-Frozen Spines.Anal Chem. 2023 Nov 28;95(47):17337-17346. doi: 10.1021/acs.analchem.3c03672. Epub 2023 Oct 27. Anal Chem. 2023. PMID: 37886878 Free PMC article.
-
Spatial quantitation of antibiotics in bone tissue compartments by laser-capture microdissection coupled with UHPLC-tandem mass spectrometry.Anal Bioanal Chem. 2022 Sep;414(23):6919-6927. doi: 10.1007/s00216-022-04257-3. Epub 2022 Aug 10. Anal Bioanal Chem. 2022. PMID: 35945288 Free PMC article.
-
Mass Spectrometry Reveals Molecular Effects of Citrulline Supplementation during Bone Fracture Healing in a Rat Model.J Am Soc Mass Spectrom. 2024 Jun 5;35(6):1184-1196. doi: 10.1021/jasms.4c00028. Epub 2024 Apr 28. J Am Soc Mass Spectrom. 2024. PMID: 38679918 Free PMC article.
-
Mass Spectrometry Imaging: Revolutionizing Molecular Insights in Infectious Diseases Research.Pathogens. 2025 Jun 30;14(7):645. doi: 10.3390/pathogens14070645. Pathogens. 2025. PMID: 40732693 Free PMC article. Review.
References
-
- Chughtai S, Chughtai K, Cillero-Pastor B, Kiss A, Agrawal P, MacAleese L, et al. A multimodal mass spectrometry imaging approach for the study of musculoskeletal tissues. Int J Mass Spectrom. 2012;325–327:150–60 Available from: http://www.sciencedirect.com/science/article/pii/S1387380612002308. Accessed 11 Sept 2020.
-
- Cohen L, Go E, Siuzdak G. Small molecule desorption-ionization mass analysis. MALDI MS. A practical guide to instrumentation, methods and applications. 2007. p. 299–337.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

