Genetic Loss of IK1 Causes Adrenergic-Induced Phase 3 Early Afterdepolariz ations and Polymorphic and Bidirectional Ventricular Tachycardia
- PMID: 32931337
- PMCID: PMC7574954
- DOI: 10.1161/CIRCEP.120.008638
Genetic Loss of IK1 Causes Adrenergic-Induced Phase 3 Early Afterdepolariz ations and Polymorphic and Bidirectional Ventricular Tachycardia
Abstract
Background: Arrhythmia syndromes associated with KCNJ2 mutations have been described clinically; however, little is known of the underlying arrhythmia mechanism. We create the first patient inspired KCNJ2 transgenic mouse and study effects of this mutation on cardiac function, IK1, and Ca2+ handling, to determine the underlying cellular arrhythmic pathogenesis.
Methods: A cardiac-specific KCNJ2-R67Q mouse was generated and bred for heterozygosity (R67Q+/-). Echocardiography was performed at rest, under anesthesia. In vivo ECG recording and whole heart optical mapping of intact hearts was performed before and after adrenergic stimulation in wild-type (WT) littermate controls and R67Q+/- mice. IK1 measurements, action potential characterization, and intracellular Ca2+ imaging from isolated ventricular myocytes at baseline and after adrenergic stimulation were performed in WT and R67Q+/- mice.
Results: R67Q+/- mice (n=17) showed normal cardiac function, structure, and baseline electrical activity compared with WT (n=10). Following epinephrine and caffeine, only the R67Q+/- mice had bidirectional ventricular tachycardia, ventricular tachycardia, frequent ventricular ectopy, and/or bigeminy and optical mapping demonstrated high prevalence of spontaneous and sustained ventricular arrhythmia. Both R67Q+/- (n=8) and WT myocytes (n=9) demonstrated typical n-shaped IK1IV relationship; however, following isoproterenol, max outward IK1 increased by ≈20% in WT but decreased by ≈24% in R67Q+/- (P<0.01). R67Q+/- myocytes (n=5) demonstrated prolonged action potential duration at 90% repolarization and after 10 nmol/L isoproterenol compared with WT (n=7; P<0.05). Ca2+ transient amplitude, 50% decay rate, and sarcoplasmic reticulum Ca2+ content were not different between WT (n=18) and R67Q+/- (n=16) myocytes. R67Q+/- myocytes (n=10) under adrenergic stimulation showed frequent spontaneous development of early afterdepolarizations that occurred at phase 3 of action potential repolarization.
Conclusions: KCNJ2 mutation R67Q+/- causes adrenergic-dependent loss of IK1 during terminal repolarization and vulnerability to phase 3 early afterdepolarizations. This model clarifies a heretofore unknown arrhythmia mechanism and extends our understanding of treatment implications for patients with KCNJ2 mutation.
Keywords: action potential; heart; potassium channels; sarcoplasmic reticulum; tachycardia, ventricular.
Figures



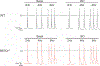


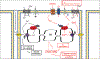
Similar articles
-
KCNJ2 mutation causes an adrenergic-dependent rectification abnormality with calcium sensitivity and ventricular arrhythmia.Heart Rhythm. 2014 May;11(5):885-94. doi: 10.1016/j.hrthm.2014.02.015. Epub 2014 Feb 21. Heart Rhythm. 2014. PMID: 24561538 Free PMC article.
-
Pharmacological Enhancement of Small Conductance Ca2+-Activated K+ Channels Suppresses Cardiac Arrhythmias in a Mouse Model of Catecholaminergic Polymorphic Ventricular Tachycardia.Circ Res. 2025 Jul 18;137(3):386-399. doi: 10.1161/CIRCRESAHA.124.325477. Epub 2025 May 29. Circ Res. 2025. PMID: 40438932
-
In situ confocal imaging in intact heart reveals stress-induced Ca(2+) release variability in a murine catecholaminergic polymorphic ventricular tachycardia model of type 2 ryanodine receptor(R4496C+/-) mutation.Circ Arrhythm Electrophysiol. 2012 Aug 1;5(4):841-9. doi: 10.1161/CIRCEP.111.969733. Epub 2012 Jun 21. Circ Arrhythm Electrophysiol. 2012. PMID: 22722659 Free PMC article.
-
Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4496C underlies catecholaminergic polymorphic ventricular tachycardia.Circ Res. 2009 Jan 30;104(2):201-9, 12p following 209. doi: 10.1161/CIRCRESAHA.108.177493. Epub 2008 Dec 18. Circ Res. 2009. PMID: 19096022 Free PMC article.
-
The network of cardiac KIR2.1: its function, cellular regulation, electrical signaling, diseases and new drug avenues.Naunyn Schmiedebergs Arch Pharmacol. 2024 Sep;397(9):6369-6389. doi: 10.1007/s00210-024-03116-5. Epub 2024 Apr 29. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38683369 Free PMC article. Review.
Cited by
-
Cardiac potassium inward rectifier Kir2: Review of structure, regulation, pharmacology, and arrhythmogenesis.Heart Rhythm. 2021 Aug;18(8):1423-1434. doi: 10.1016/j.hrthm.2021.04.008. Epub 2021 Apr 20. Heart Rhythm. 2021. PMID: 33857643 Free PMC article. Review.
-
Atomic-level investigation of KCNJ2 mutations associated with ventricular arrhythmic syndrome phenotypes.Sci Rep. 2025 Apr 2;15(1):11290. doi: 10.1038/s41598-025-95062-2. Sci Rep. 2025. PMID: 40175568 Free PMC article.
-
Caveolin-3 prevents swelling-induced membrane damage via regulation of ICl,swell activity.Biophys J. 2022 May 3;121(9):1643-1659. doi: 10.1016/j.bpj.2022.04.001. Epub 2022 Apr 2. Biophys J. 2022. PMID: 35378081 Free PMC article.
-
Illuminating Kir channel function in Anderson-Tawil syndrome.Cardiovasc Res. 2021 Jul 7;117(8):1803-1805. doi: 10.1093/cvr/cvab062. Cardiovasc Res. 2021. PMID: 33629095 Free PMC article. No abstract available.
-
Isolated Perfused Hearts for Cardiovascular Research: An Old Dog with New Tricks.J Cardiovasc Transl Res. 2024 Oct;17(5):1207-1217. doi: 10.1007/s12265-024-10517-7. Epub 2024 May 8. J Cardiovasc Transl Res. 2024. PMID: 38717725 Free PMC article. Review.
References
-
- Willis BC, Pandit S, Ponce-Balbuena D, Zarzoso M, Guerrero-Serna G, Limbu B, Deo M, Cammors E, Ramirez RJ, Mironov S, et al. Constitutive Intracellular Na +Excess in Purkinje Cells Promotes Arrhythmogenesis at Lower Levels of Stress than Ventricular Myocytes from Mice with Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation. 2016;133:CIRCULATIONAHA.116.021936 57. Available from: 10.1161/circulationaha.116.021936 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

