Prospects for mucosal vaccine: shutting the door on SARS-CoV-2
- PMID: 32931361
- PMCID: PMC7544966
- DOI: 10.1080/21645515.2020.1805992
Prospects for mucosal vaccine: shutting the door on SARS-CoV-2
Abstract
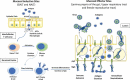
The sudden emergence of a highly transmissible and pathogenic coronavirus SARS-CoV-2 in December 2019 from China and its rapid global spread has posed an international health emergency. The rapid development of an effective vaccine is imperative to control the spread of SARS-CoV-2. A number of concurrent efforts to find an effective therapeutic agent or vaccine for COVID-19 (coronavirus disease 2019) are being undertaken globally. Oral and nasal mucosal surfaces serve as the primary portal of entry for pathogens like coronaviruses in the human body. As evidenced by studies on similar coronaviruses (SARS-CoV and MERS-CoV), mucosal vaccination can provide a safe and effective means for the induction of long-lasting systemic and mucosal immunity to confer protection against SARS-CoV-2. This article summarizes the approaches to an effective mucosal vaccine formulation which can be a rewarding approach to combat the unprecedented threat posed by this emerging global pandemic.
Keywords: Coronavirus; Covid-19; SARS-CoV-2; mucosal vaccine; vaccine.
Figures

References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM.. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
