Fbxo2 mediates clearance of damaged lysosomes and modifies neurodegeneration in the Niemann-Pick C brain
- PMID: 32931479
- PMCID: PMC7605537
- DOI: 10.1172/jci.insight.136676
Fbxo2 mediates clearance of damaged lysosomes and modifies neurodegeneration in the Niemann-Pick C brain
Abstract
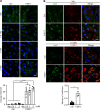
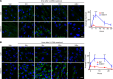
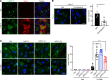
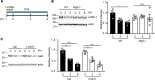
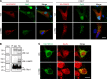
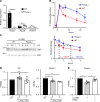
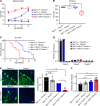
A critical response to lysosomal membrane permeabilization (LMP) is the clearance of damaged lysosomes through a selective form of macroautophagy known as lysophagy. Although regulators of this process are emerging, whether organ- and cell-specific components contribute to the control of lysophagy remains incompletely understood. Here, we examined LMP and lysophagy in Niemann-Pick type C (NPC) disease, an autosomal recessive disorder characterized by the accumulation of unesterified cholesterol within late endosomes and lysosomes, leading to neurodegeneration and early death. We demonstrated that NPC human fibroblasts show enhanced sensitivity to lysosomal damage as a consequence of lipid storage. Moreover, we described a role for the glycan-binding F-box protein 2 (Fbxo2) in CNS lysophagy. Fbxo2 functions as a component of the S phase kinase-associated protein 1-cullin 1-F-box protein (SKP1-CUL1-SCF) ubiquitin ligase complex. Loss of Fbxo2 in mouse primary cortical cultures delayed clearance of damaged lysosomes and decreased viability after lysosomal damage. Moreover, Fbxo2 deficiency in a mouse model of NPC exacerbated deficits in motor function, enhanced neurodegeneration, and reduced survival. Collectively, our data identified a role for Fbxo2 in CNS lysophagy and establish its functional importance in NPC.
Keywords: Autophagy; Lysosomes; Neurodegeneration; Neuroscience.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

