Biannual azithromycin distribution and child mortality among malnourished children: A subgroup analysis of the MORDOR cluster-randomized trial in Niger
- PMID: 32931496
- PMCID: PMC7491708
- DOI: 10.1371/journal.pmed.1003285
Biannual azithromycin distribution and child mortality among malnourished children: A subgroup analysis of the MORDOR cluster-randomized trial in Niger
Abstract
Background: Biannual azithromycin distribution has been shown to reduce child mortality as well as increase antimicrobial resistance. Targeting distributions to vulnerable subgroups such as malnourished children is one approach to reaching those at the highest risk of mortality while limiting selection for resistance. The objective of this analysis was to assess whether the effect of azithromycin on mortality differs by nutritional status.
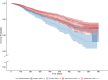
Methods and findings: A large simple trial randomized communities in Niger to receive biannual distributions of azithromycin or placebo to children 1-59 months old over a 2-year timeframe. In exploratory subgroup analyses, the effect of azithromycin distribution on child mortality was assessed for underweight subgroups using weight-for-age Z-score (WAZ) thresholds of -2 and -3. Modification of the effect of azithromycin on mortality by underweight status was examined on the additive and multiplicative scale. Between December 2014 and August 2017, 27,222 children 1-11 months of age from 593 communities had weight measured at their first study visit. Overall, the average age among included children was 4.7 months (interquartile range [IQR] 3-6), 49.5% were female, 23% had a WAZ < -2, and 10% had a WAZ < -3. This analysis included 523 deaths in communities assigned to azithromycin and 661 deaths in communities assigned to placebo. The mortality rate was lower in communities assigned to azithromycin than placebo overall, with larger reductions among children with lower WAZ: -12.6 deaths per 1,000 person-years (95% CI -18.5 to -6.9, P < 0.001) overall, -17.0 (95% CI -28.0 to -7.0, P = 0.001) among children with WAZ < -2, and -25.6 (95% CI -42.6 to -9.6, P = 0.003) among children with WAZ < -3. No statistically significant evidence of effect modification was demonstrated by WAZ subgroup on either the additive or multiplicative scale (WAZ < -2, additive: 95% CI -6.4 to 16.8, P = 0.34; WAZ < -2, multiplicative: 95% CI 0.8 to 1.4, P = 0.50, WAZ < -3, additive: 95% CI -2.2 to 31.1, P = 0.14; WAZ < -3, multiplicative: 95% CI 0.9 to 1.7, P = 0.26). The estimated number of deaths averted with azithromycin was 388 (95% CI 214 to 574) overall, 116 (95% CI 48 to 192) among children with WAZ < -2, and 76 (95% CI 27 to 127) among children with WAZ < -3. Limitations include the availability of a single weight measurement on only the youngest children and the lack of power to detect small effect sizes with this rare outcome. Despite the trial's large size, formal tests for effect modification did not reach statistical significance at the 95% confidence level.
Conclusions: Although mortality rates were higher in the underweight subgroups, this study was unable to demonstrate that nutritional status modified the effect of biannual azithromycin distribution on mortality. Even if the effect were greater among underweight children, a nontargeted intervention would result in the greatest absolute number of deaths averted.
Trial registration: The MORDOR trial is registered at clinicaltrials.gov NCT02047981.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
Priorities in reducing child mortality: Azithromycin and other interventions.PLoS Med. 2020 Sep 15;17(9):e1003364. doi: 10.1371/journal.pmed.1003364. eCollection 2020 Sep. PLoS Med. 2020. PMID: 32931499 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

