Frustrated Radical Pairs: Insights from EPR Spectroscopy
- PMID: 32931604
- PMCID: PMC7883636
- DOI: 10.1002/anie.202010633
Frustrated Radical Pairs: Insights from EPR Spectroscopy
Abstract
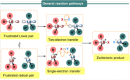
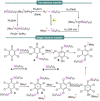
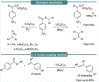
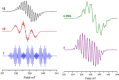
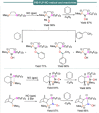
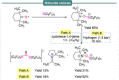
Progress in frustrated Lewis pair (FLP) chemistry has revealed the importance of the main group elements in catalysis, opening new avenues in synthetic chemistry. Recently, new reactivities of frustrated Lewis pairs have been uncovered that disclose that certain combinations of Lewis acids and bases undergo single-electron transfer (SET) processes. Here an electron can be transferred from the Lewis basic donor to a Lewis acidic acceptor to generate a reactive frustrated radical pair (FRP). This minireview aims to showcase the recent advancements in this emerging field covering the synthesis and reactivities of frustrated radical pairs, with extensive highlights of the results from Electron Paramagnetic Resonance (EPR) spectroscopy to explain the nature and stability of the different radical species observed.
Keywords: electron paramagnetic resonance; frustrated Lewis pair; main group chemistry; radicals; single-electron transfer.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




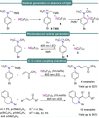
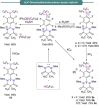

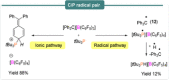

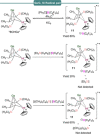


References
-
- For reviews see:
-
- Lam J., Szkop K. M., Mosaferi E., Stephan D. W., Chem. Soc. Rev. 2019, 48, 3592–3612; - PubMed
-
- Jupp A. R., Stephan D. W., Trends Chem. 2019, 1, 35–48;
-
- Liu L., Lukose B., Jaque P., Ensing B., Green Energy Environ. 2019, 4, 20–28;
-
- Scott D. J., Fuchter M. J., Ashley A. E., Chem. Soc. Rev. 2017, 46, 5689–5700; - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

