Hydrogel Network Dynamics Regulate Vascular Morphogenesis
- PMID: 32931729
- PMCID: PMC7655724
- DOI: 10.1016/j.stem.2020.08.005
Hydrogel Network Dynamics Regulate Vascular Morphogenesis
Abstract
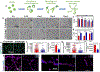
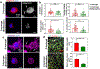
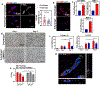
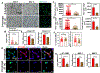
Matrix dynamics influence how individual cells develop into complex multicellular tissues. Here, we develop hydrogels with identical polymer components but different crosslinking capacities to enable the investigation of mechanisms underlying vascular morphogenesis. We show that dynamic (D) hydrogels increase the contractility of human endothelial colony-forming cells (hECFCs), promote the clustering of integrin β1, and promote the recruitment of vinculin, leading to the activation of focal adhesion kinase (FAK) and metalloproteinase expression. This leads to the robust assembly of vasculature and the deposition of new basement membrane. We also show that non-dynamic (N) hydrogels do not promote FAK signaling and that stiff D- and N-hydrogels are constrained for vascular morphogenesis. Furthermore, D-hydrogels promote hECFC microvessel formation and angiogenesis in vivo. Our results indicate that cell contractility mediates integrin signaling via inside-out signaling and emphasizes the importance of matrix dynamics in vascular tissue formation, thus informing future studies of vascularization and tissue engineering applications.
Keywords: cell contractility; integrin clustering; stress-relaxation; vasculogenesis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



Comment in
-
Dynamic Hydrogels for Investigating Vascularization.Cell Stem Cell. 2020 Nov 5;27(5):697-698. doi: 10.1016/j.stem.2020.10.009. Cell Stem Cell. 2020. PMID: 33157044
References
-
- Beamish JA, Juliar BA, Cleveland DS, Busch ME, Nimmagadda L, and Putnam AJ (2019). Deciphering the relative roles of matrix metalloproteinase-and plasmin-mediated matrix degradation during capillary morphogenesis using engineered hydrogels. Journal of Biomedical Materials Research Part B: Applied Biomaterials. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

