A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2
- PMID: 32931734
- PMCID: PMC7437481
- DOI: 10.1016/j.cell.2020.08.026
A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2
Abstract
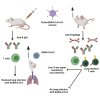
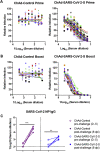
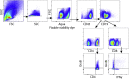
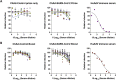
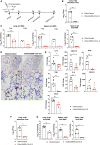
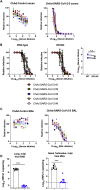
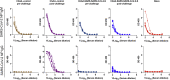
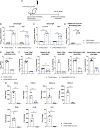
The coronavirus disease 2019 pandemic has made deployment of an effective vaccine a global health priority. We evaluated the protective activity of a chimpanzee adenovirus-vectored vaccine encoding a prefusion stabilized spike protein (ChAd-SARS-CoV-2-S) in challenge studies with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and mice expressing the human angiotensin-converting enzyme 2 receptor. Intramuscular dosing of ChAd-SARS-CoV-2-S induces robust systemic humoral and cell-mediated immune responses and protects against lung infection, inflammation, and pathology but does not confer sterilizing immunity, as evidenced by detection of viral RNA and induction of anti-nucleoprotein antibodies after SARS-CoV-2 challenge. In contrast, a single intranasal dose of ChAd-SARS-CoV-2-S induces high levels of neutralizing antibodies, promotes systemic and mucosal immunoglobulin A (IgA) and T cell responses, and almost entirely prevents SARS-CoV-2 infection in both the upper and lower respiratory tracts. Intranasal administration of ChAd-SARS-CoV-2-S is a candidate for preventing SARS-CoV-2 infection and transmission and curtailing pandemic spread.
Keywords: COVID-19; IgA; SARS-CoV-2; T cells; antibody; intranasal; mucosal immunity; pathogenesis; protection; vaccine.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests M.S.D. is a consultant for Inbios, Vir Biotechnology, and NGM Biopharmaceuticals and on the Scientific Advisory Board of Moderna. The Diamond laboratory has received unrelated funding support from Moderna, Vir Biotechnology, and Emergent BioSolutions. M.S.D., D.T.C., A.O.H., and I.P.D. have filed a disclosure with Washington University for possible development of ChAd-SARS-CoV-2. M.J.H. is a member of the DSMB for AstraZeneca and founder of NuPeak Therapeutics. The Baric laboratory has received unrelated funding support from Takeda, Pfizer, and Eli Lilly.
Figures






References
-
- Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., Baric R.S. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85:12201–12215. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

