Cell-Autonomous versus Systemic Akt Isoform Deletions Uncovered New Roles for Akt1 and Akt2 in Breast Cancer
- PMID: 32931746
- PMCID: PMC8291754
- DOI: 10.1016/j.molcel.2020.08.017
Cell-Autonomous versus Systemic Akt Isoform Deletions Uncovered New Roles for Akt1 and Akt2 in Breast Cancer
Abstract
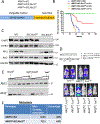
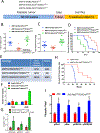
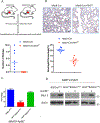
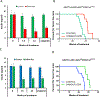
Studies in three mouse models of breast cancer identified profound discrepancies between cell-autonomous and systemic Akt1- or Akt2-inducible deletion on breast cancer tumorigenesis and metastasis. Although systemic Akt1 deletion inhibits metastasis, cell-autonomous Akt1 deletion does not. Single-cell mRNA sequencing revealed that systemic Akt1 deletion maintains the pro-metastatic cluster within primary tumors but ablates pro-metastatic neutrophils. Systemic Akt1 deletion inhibits metastasis by impairing survival and mobilization of tumor-associated neutrophils. Importantly, either systemic or neutrophil-specific Akt1 deletion is sufficient to inhibit metastasis of Akt-proficient tumors. Thus, Akt1-specific inhibition could be therapeutic for breast cancer metastasis regardless of primary tumor origin. Systemic Akt2 deletion does not inhibit and exacerbates mammary tumorigenesis and metastasis, but cell-autonomous Akt2 deletion prevents breast cancer tumorigenesis by ErbB2. Elevated circulating insulin level induced by Akt2 systemic deletion hyperactivates tumor Akt, exacerbating ErbB2-mediated tumorigenesis, curbed by pharmacological reduction of the elevated insulin.
Keywords: Akt1; Akt2; breast cancer; insulin; metastasis; neutrophils; therapy.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



References
-
- Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta KJ, Bader JS, and Ewald AJ (2016). Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A 113, E854–863. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

