Pregnancy-Related Hormones Increase Nifedipine Metabolism in Human Hepatocytes by Inducing CYP3A4 Expression
- PMID: 32931777
- PMCID: PMC7750305
- DOI: 10.1016/j.xphs.2020.09.013
Pregnancy-Related Hormones Increase Nifedipine Metabolism in Human Hepatocytes by Inducing CYP3A4 Expression
Abstract
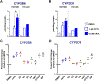
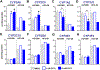
Pregnancy-related hormones (PRH) have emerged as key regulators of hepatic cytochrome P450 (CYP) enzyme expression and function. The impact of PRH on protein levels of CYP3A4 and other key CYP enzymes, and the metabolism of nifedipine (a CYP3A4 substrate commonly prescribed during pregnancy), was evaluated in primary human hepatocytes. Sandwich-cultured human hepatocytes (SCHH) from female donors were exposed to PRH (estradiol, estriol, estetrol, progesterone, and cortisol), individually or in combination as a cocktail. Absolute protein concentrations of twelve CYP isoforms in SCHH membrane fractions were quantified by nanoLC-MS/MS, and metabolism of nifedipine to dehydronifedipine in SCHH was evaluated. PRH significantly increased CYP3A4 protein concentrations and nifedipine metabolism to dehydronifedipine in a concentration-dependent manner. CYP3A4 mRNA levels in hepatocyte-derived exosomes positively correlated with CYP3A4 protein levels and dehydronifedipine formation in SCHH. PRH also increased CYP2B6, CYP2C8 and CYP2A6 levels. Our findings demonstrate that PRH increase nifedipine metabolism in SCHH by inducing CYP3A4 expression and alter expression of other key CYP proteins in an isoform-specific manner, and suggest that hepatocyte-derived exosomes warrant further investigation as biomarkers of hepatic CYP3A4 metabolism. Together, these results offer mechanistic insight into the increases in nifedipine metabolism and clearance observed in pregnant women.
Keywords: Cytochrome P450; Estradiol; Exosomes; Hepatic metabolism; Hypertension; Nifedipine; Pregnancy; Progesterone; Targeted proteomics.
Copyright © 2020 American Pharmacists Association®. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
Dr. Kim Brouwer is a co-inventor of the sandwich-cultured hepatocyte technology for quantification of biliary excretion (B-CLEAR®) and related technologies, which have been licensed exclusively to Qualyst Transporter Solutions, recently acquired by BioIVT. The other authors have no conflicts of interest to disclose.
Figures




References
-
- Tasnif Y, Morado J, Hebert MF. Pregnancy-Related Pharmacokinetic Changes. Clin Pharmacol Ther 2016;100(1):53–62. - PubMed
-
- Dallmann A, Pfister M, van den Anker J, Eissing T. Physiologically Based Pharmacokinetic Modeling in Pregnancy: A Systematic Review of Published Models. Clin Pharmacol Ther 2018;104(6):1110–1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

