Biological effects of inhaled hydraulic fracturing sand dust. VI. Cardiovascular effects
- PMID: 32931794
- PMCID: PMC7673239
- DOI: 10.1016/j.taap.2020.115242
Biological effects of inhaled hydraulic fracturing sand dust. VI. Cardiovascular effects
Abstract
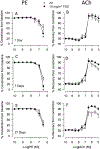
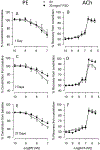
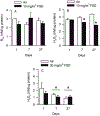
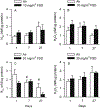
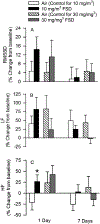
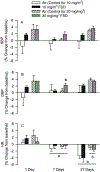
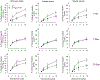
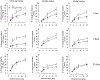
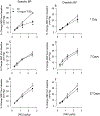
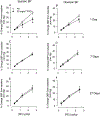
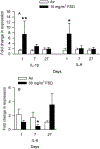
Hydraulic fracturing is used to access oil and natural gas reserves. This process involves the high-pressure injection of fluid to fracture shale. Fracking fluid contains approximately 95% water, chemicals and 4.5% fracking sand. Workers may be exposed to fracking sand dust (FSD) during the manipulation of the sand, and negative health consequences could occur if FSD is inhaled. In the absence of any information about its potential toxicity, a comprehensive rat animal model study (see Fedan et al., 2020) was designed to investigate the bioactivities of several FSDs in comparison to MIN-U-SIL® 5, a respirable α-quartz reference dust used in previous animal models of silicosis, in several organ systems. The goal of this study was to assess the effects of inhalation of one FSD, i.e., FSD 8, on factors and tissues that affect cardiovascular function. Male rats were exposed to 10 or 30 mg/m3 FSD (6 h/d for 4 d) by whole body inhalation, with measurements made 1, 7 or 27 d post-exposure. One day following exposure to 10 mg/m3 FSD the sensitivity to phenylephrine-induced vasoconstriction in tail arteries in vitro was increased. FSD exposure at both doses resulted in decreases in heart rate (HR), HR variability, and blood pressure in vivo. FSD induced changes in hydrogen peroxide concentrations and transcript levels for pro-inflammatory factors in heart tissues. In kidney, expression of proteins indicative of injury and remodeling was reduced after FSD exposure. When analyzed using regression analysis, changes in proteins involved in repair and remodeling were correlated. Thus, it appears that inhalation of FSD does have some prolonged effects on cardiovascular, and, possibly, renal function. The findings also provide information regarding potential mechanisms that may lead to these changes, and biomarkers that could be examined to monitor physiological changes that could be indicative of impending cardiovascular dysfunction.
Keywords: Blood pressure; Heart rate variability; Oxidative stress; Renal injury; Vasoconstriction; Vasodilation.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no conflicts of interest in relation to this publication.
Figures


References
-
- Anderson SE, Shane H, Long C, Marrocco A, Lukomska E, Roberts JR, Marshall N, Fedan JS, 2020. Biological effects of inhaled hydraulic fracturing sand dust. VIII. Immunotoxicity. Toxicol. Appl. Pharmacol (Manuscript submitted to this journal as a tandem paper to accompany this manuscript.). - PMC - PubMed
-
- Cauley E, Wang X, Dyavanapalli J, Sun K, Garrott K, Kuzmiak-Glancy S, Kay MW, Mendelowitz D, 2015. Neurotransmission to parasympathetic cardiac vagal neurons in the brain stem is altered with left ventricular hypertrophy-induced heart failure. Am. J. Physiol. Heart Circ. Physiol 309, H1281–H1287. - PMC - PubMed
-
- Esswein E, Kiefer M, Snawder J, Breitenstein M, 2012. Worker exposure to crystalline silica during hydraulic fracturing. In: NIOSH/CDC. NIOSH Science Blog. http://blogs.cdc.gov/niosh-science-blog/2012/05/23/silica-fracking. - PubMed
-
- Esswein EJ, Breitenstein M, Snawder J, Kiefer M, Sieber WK, 2013. Occupational exposures to respirable crystalline silica during hydraulic fracturing. J. Occup. Environ. Hyg 10, 347–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

