Cancer-Associated Fibroblasts Provide a Stromal Niche for Liver Cancer Organoids That Confers Trophic Effects and Therapy Resistance
- PMID: 32932015
- PMCID: PMC7788239
- DOI: 10.1016/j.jcmgh.2020.09.003
Cancer-Associated Fibroblasts Provide a Stromal Niche for Liver Cancer Organoids That Confers Trophic Effects and Therapy Resistance
Abstract
Background & aims: Cancer-associated fibroblasts (CAFs) play a key role in the cancer process, but the research progress is hampered by the paucity of preclinical models that are essential for mechanistic dissection of cancer cell-CAF interactions. Here, we aimed to establish 3-dimensional (3D) organotypic co-cultures of primary liver tumor-derived organoids with CAFs, and to understand their interactions and the response to treatment.
Methods: Liver tumor organoids and CAFs were cultured from murine and human primary liver tumors. 3D co-culture models of tumor organoids with CAFs and Transwell culture systems were established in vitro. A xenograft model was used to investigate the cell-cell interactions in vivo. Gene expression analysis of CAF markers in our hepatocellular carcinoma cohort and an online liver cancer database indicated the clinical relevance of CAFs.
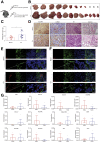
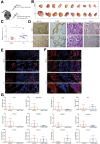
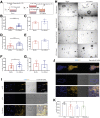
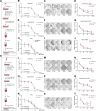
Results: To functionally investigate the interactions of liver cancer cells with CAFs, we successfully established murine and human 3D co-culture models of liver tumor organoids with CAFs. CAFs promoted tumor organoid growth in co-culture with direct cell-cell contact and in a Transwell system via paracrine signaling. Vice versa, cancer cells secrete paracrine factors regulating CAF physiology. Co-transplantation of CAFs with liver tumor organoids of mouse or human origin promoted tumor growth in xenograft models. Moreover, tumor organoids conferred resistance to clinically used anticancer drugs including sorafenib, regorafenib, and 5-fluorouracil in the presence of CAFs, or the conditioned medium of CAFs.
Conclusions: We successfully established murine and human 3D co-culture models and have shown robust effects of CAFs in liver cancer nurturing and treatment resistance.
Keywords: Cell–Cell Contact; Co-Culture; Liver Tumor Organoids; Paracrine Effect; Stromal Cells.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Cancer-associated fibroblasts nurture LGR5 marked liver tumor-initiating cells and promote their tumor formation, growth, and metastasis.Cancer Med. 2023 Sep;12(17):18032-18049. doi: 10.1002/cam4.6408. Epub 2023 Aug 14. Cancer Med. 2023. PMID: 37578396 Free PMC article.
-
Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP.J Exp Clin Cancer Res. 2019 Apr 18;38(1):170. doi: 10.1186/s13046-019-1163-6. J Exp Clin Cancer Res. 2019. PMID: 30999932 Free PMC article.
-
The BAFF/NFκB axis is crucial to interactions between sorafenib-resistant HCC cells and cancer-associated fibroblasts.Cancer Sci. 2021 Sep;112(9):3545-3554. doi: 10.1111/cas.15041. Epub 2021 Jul 16. Cancer Sci. 2021. PMID: 34159680 Free PMC article.
-
Emerging Role of Cancer-Associated Fibroblasts in Progression and Treatment of Hepatocellular Carcinoma.Int J Mol Sci. 2023 Feb 15;24(4):3941. doi: 10.3390/ijms24043941. Int J Mol Sci. 2023. PMID: 36835352 Free PMC article. Review.
-
Organoids as research models for hepatocellular carcinoma.Exp Cell Res. 2022 Feb 1;411(1):112987. doi: 10.1016/j.yexcr.2021.112987. Epub 2021 Dec 21. Exp Cell Res. 2022. PMID: 34942189 Review.
Cited by
-
Patient-derived tumor organoids: a new avenue for preclinical research and precision medicine in oncology.Exp Mol Med. 2024 Jul;56(7):1531-1551. doi: 10.1038/s12276-024-01272-5. Epub 2024 Jul 1. Exp Mol Med. 2024. PMID: 38945959 Free PMC article. Review.
-
Complex Analysis of Single-Cell RNA Sequencing Data.Biochemistry (Mosc). 2023 Feb;88(2):231-252. doi: 10.1134/S0006297923020074. Biochemistry (Mosc). 2023. PMID: 37072324 Free PMC article. Review.
-
Drug Discovery in Liver Disease Using Kinome Profiling.Int J Mol Sci. 2021 Mar 5;22(5):2623. doi: 10.3390/ijms22052623. Int J Mol Sci. 2021. PMID: 33807722 Free PMC article. Review.
-
3D Cancer Models: Depicting Cellular Crosstalk within the Tumour Microenvironment.Cancers (Basel). 2021 Sep 14;13(18):4610. doi: 10.3390/cancers13184610. Cancers (Basel). 2021. PMID: 34572836 Free PMC article. Review.
-
Inactivation of ERK1/2 in cancer-associated hepatic stellate cells suppresses cancer-stromal interaction by regulating extracellular matrix in fibrosis.Am J Cancer Res. 2024 Mar 15;14(3):1015-1032. doi: 10.62347/VPYE3817. eCollection 2024. Am J Cancer Res. 2024. PMID: 38590418 Free PMC article.
References
-
- Batlle E., Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–1134. - PubMed
-
- Tuveson D., Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364:952–955. - PubMed
-
- Broutier L., Mastrogiovanni G., Verstegen M.M., Francies H.E., Gavarro L.M., Bradshaw C.R., Allen G.E., Arnes-Benito R., Sidorova O., Gaspersz M.P., Georgakopoulos N., Koo B.K., Dietmann S., Davies S.E., Praseedom R.K., Lieshout R., JNM I.J., Wigmore S.J., Saeb-Parsy K., Garnett M.J., van der Laan L.J., Huch M. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. - PMC - PubMed
-
- Cao W., Liu J., Wang L., Li M., Verstegen M.M.A., Yin Y., Ma B., Chen K., Bolkestein M., Sprengers D., van der Laan L.J.W., Doukas M., Kwekkeboom J., Smits R., Peppelenbosch M.P., Pan Q. Modeling liver cancer and therapy responsiveness using organoids derived from primary mouse liver tumors. Carcinogenesis. 2019;40:145–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

