Aryl Hydrocarbon Receptor Activity in Hepatocytes Sensitizes to Hyperacute Acetaminophen-Induced Hepatotoxicity in Mice
- PMID: 32932016
- PMCID: PMC7779786
- DOI: 10.1016/j.jcmgh.2020.09.002
Aryl Hydrocarbon Receptor Activity in Hepatocytes Sensitizes to Hyperacute Acetaminophen-Induced Hepatotoxicity in Mice
Abstract
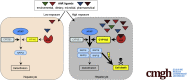
Background & aims: Acetaminophen (APAP)-induced liver injury is one of the most common causes of acute liver failure, however, a clear definition of sensitizing risk factors is lacking. Here, we investigated the role of the ligand-activated transcription factor aryl hydrocarbon receptor (Ahr) in APAP-induced liver injury. We hypothesized that Ahr, which integrates environmental, dietary, microbial and metabolic signals into complex cellular transcriptional programs, might act as a rheostat for APAP-toxicity.
Methods: Wildtype or conditional Ahr knockout mice lacking Ahr in hepatocytes (AlbΔ/ΔAhr) or myeloid cells (LysMΔ/ΔAhr) were treated with the specific Ahr ligand 2-(1'H-indole-3'-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) together with APAP.
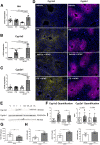
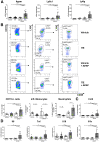
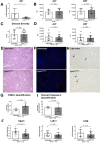
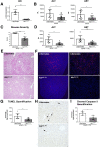
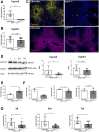
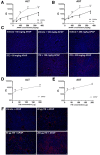
Results: Ahr activation by ITE, which by itself was non-toxic, exacerbated APAP-induced hepatotoxicity compared to vehicle-treated controls, causing 80% vs. 0% mortality after administration of a normally sublethal APAP overdose. Of note, Ahr activation induced hepatocyte death even at APAP doses within the therapeutic range. Aggravated liver injury was associated with significant neutrophil infiltration; however, lack of Ahr in myeloid cells did not protect LysMΔ/ΔAhr mice from exacerbated APAP hepatotoxicity. In contrast, AlbΔ/ΔAhr mice were largely protected from ITE-induced aggravated liver damage, indicating that Ahr activation in hepatocytes, but not in myeloid cells, was instrumental for disease exacerbation. Mechanistically, Ahr activation fueled hepatic accumulation of toxic APAP metabolites by up-regulating expression of the APAP-metabolizing enzyme Cyp1a2, a direct Ahr downstream target.
Conclusions: Ahr activation in hepatocytes potentiates APAP-induced hepatotoxicity. Thus, individual exposition to environmental Ahr ligands might explain individual sensitivity to hyperacute liver failure.
Keywords: APAP; Acute Liver Failure; Ahr; Cyp1a2.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Aryl Hydrocarbon Receptor Signaling Prevents Activation of Hepatic Stellate Cells and Liver Fibrogenesis in Mice.Gastroenterology. 2019 Sep;157(3):793-806.e14. doi: 10.1053/j.gastro.2019.05.066. Epub 2019 Jun 3. Gastroenterology. 2019. PMID: 31170413 Free PMC article.
-
Activation of Aryl Hydrocarbon Receptor by ITE Improves Cardiac Function in Mice After Myocardial Infarction.J Am Heart Assoc. 2021 Jul 6;10(13):e020502. doi: 10.1161/JAHA.120.020502. Epub 2021 Jun 23. J Am Heart Assoc. 2021. PMID: 34157850 Free PMC article.
-
Induction of a chloracne phenotype in an epidermal equivalent model by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is dependent on aryl hydrocarbon receptor activation and is not reproduced by aryl hydrocarbon receptor knock down.J Dermatol Sci. 2014 Jan;73(1):10-22. doi: 10.1016/j.jdermsci.2013.09.001. Epub 2013 Sep 11. J Dermatol Sci. 2014. PMID: 24161567 Free PMC article.
-
Acetaminophen: Dose-Dependent Drug Hepatotoxicity and Acute Liver Failure in Patients.Dig Dis. 2015;33(4):464-71. doi: 10.1159/000374090. Epub 2015 Jul 6. Dig Dis. 2015. PMID: 26159260 Free PMC article. Review.
-
Novel strategies for the treatment of acetaminophen hepatotoxicity.Expert Opin Drug Metab Toxicol. 2020 Nov;16(11):1039-1050. doi: 10.1080/17425255.2020.1817896. Epub 2020 Sep 14. Expert Opin Drug Metab Toxicol. 2020. PMID: 32862728 Free PMC article. Review.
Cited by
-
Spatio-Temporal Multiscale Analysis of Western Diet-Fed Mice Reveals a Translationally Relevant Sequence of Events during NAFLD Progression.Cells. 2021 Sep 23;10(10):2516. doi: 10.3390/cells10102516. Cells. 2021. PMID: 34685496 Free PMC article.
-
Microbial metabolites indole derivatives sensitize mice to D-GalN/LPS induced-acute liver failure via the Tlr2/NF-κB pathway.Front Microbiol. 2023 Jan 6;13:1103998. doi: 10.3389/fmicb.2022.1103998. eCollection 2022. Front Microbiol. 2023. PMID: 36687651 Free PMC article.
-
Glutaredoxin-1 alleviates acetaminophen-induced liver injury by decreasing its toxic metabolites.J Pharm Anal. 2023 Dec;13(12):1548-1561. doi: 10.1016/j.jpha.2023.08.004. Epub 2023 Aug 9. J Pharm Anal. 2023. PMID: 38223455 Free PMC article.
-
The aryl hydrocarbon receptor in liver inflammation.Semin Immunopathol. 2021 Aug;43(4):563-575. doi: 10.1007/s00281-021-00867-8. Epub 2021 Jun 1. Semin Immunopathol. 2021. PMID: 34075438 Free PMC article. Review.
-
Acetaminophen overdose causes a breach of the blood-bile barrier in mice but not in rats.Arch Toxicol. 2024 May;98(5):1533-1542. doi: 10.1007/s00204-024-03705-6. Epub 2024 Mar 11. Arch Toxicol. 2024. PMID: 38466352
References
-
- Zaher H., Buters J.T., Ward J.M., Bruno M.K., Lucas A.M., Stern S.T., Cohen S.D., Gonzalez F.J. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol. 1998;152:193–199. - PubMed
-
- Herndon C.M., Dankenbring D.M. Patient perception and knowledge of acetaminophen in a large family medicine service. J Pain Palliat Care Pharmacother. 2014;28:109–116. - PubMed
-
- Gulmez S.E., Larrey D., Pageaux G.P., Lignot S., Lassalle R., Jové J., Gatta A., McCormick P.A., Metselaar H.J., Monteiro E., Thorburn D., Bernal W., Zouboulis-Vafiadis I., de Vries C., Perez-Gutthann S., Sturkenboom M., Bénichou J., Montastruc J.L., Horsmans Y., Salvo F., Hamoud F., Micon S., Droz-Perroteau C., Blin P., Moore N. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case-population SALT study. Drug Saf. 2013;36:135–144. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

