Evaluation of the Pharmacokinetic Drug-Drug Interaction between Micronized Fenofibrate and Pitavastatin in Healthy Volunteers
- PMID: 32932576
- PMCID: PMC7557955
- DOI: 10.3390/pharmaceutics12090869
Evaluation of the Pharmacokinetic Drug-Drug Interaction between Micronized Fenofibrate and Pitavastatin in Healthy Volunteers
Abstract
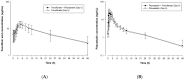
Dyslipidemia is a major risk factor for development of atherosclerosis and cardiovascular disease (CVD). Effective lipid-lowering therapies has led to CVD risk reduction. This study evaluated the possible pharmacokinetic interactions between fenofibrate, a peroxisome proliferators-activated receptors α agonist, and pitavastatin, a 3-hydoxy-3-methylglutaryl-coenzyme A reductase inhibitor, in healthy Korean subjects. The study design was an open-label, randomized, multiple-dose, three-period, and six-sequence crossover study with a 10-day washout in 24 healthy volunteers. It had three treatments: 160 mg of micronized fenofibrate once daily for 5 days; 2 mg of pitavastatin once daily for 5 days; and 160 mg of micronized fenofibrate with 2 mg of pitavastatin for 5 days. Serial blood samples were collected at scheduled intervals for up to 48 h after the last dose in each period to determine the steady-state pharmacokinetics of both drugs. Plasma concentrations of fenofibric acid and pitavastatin were measured using a validated high-performance liquid chromatography with the tandem mass spectrometry method. A total of 24 subjects completed the study. Pitavastatin, when co-administered with micronized fenofibrate, had no effect on the Cmax,ss and AUCτ,ss of fenofibric acid. The Cmax,ss and AUCτ,ss of pitavastatin were increased by 36% and 12%, respectively, when co-administered with fenofibrate. Combined treatment with pitavastatin and micronized fenofibrate was generally well tolerated without serious adverse events. Our results demonstrated no clinically significant pharmacokinetic interactions between micronized fenofibrate and pitavastatin when 160 mg of micronized fenofibrate and 2 mg of pitavastatin are co-administered. The treatments were well tolerated during the study, with no serious adverse events.
Keywords: drug-drug interaction; micronized fenofibrate; pharmacokinetics; pitavastatin; safety.
Conflict of interest statement
The authors declare no conflict of interest. Kyunghee Cho is from Biocore Co. Ltd. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Jacobson T.A., Ito M.K., Maki K.C., Orringer C.E., Bays H.E., Jones P.H., McKenney J.M., Grundy S.M., Gill E.A., Wild R.A., et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—executive summary. J. Clin. Lipidol. 2014;8:473–488. doi: 10.1016/j.jacl.2014.07.007. - DOI - PubMed
-
- Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., Goldberg A.C., Gordon D., Levy D., Llyod-Jones D.M., et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63:2889–2934. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

