Selective Apheresis of C-Reactive Protein for Treatment of Indications with Elevated CRP Concentrations
- PMID: 32932587
- PMCID: PMC7564224
- DOI: 10.3390/jcm9092947
Selective Apheresis of C-Reactive Protein for Treatment of Indications with Elevated CRP Concentrations
Abstract
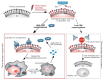
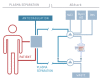
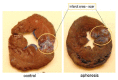
Almost every kind of inflammation in the human body is accompanied by rising C-reactive protein (CRP) concentrations. This can include bacterial and viral infection, chronic inflammation and so-called sterile inflammation triggered by (internal) acute tissue injury. CRP is part of the ancient humoral immune response and secreted into the circulation by the liver upon respective stimuli. Its main immunological functions are the opsonization of biological particles (bacteria and dead or dying cells) for their clearance by macrophages and the activation of the classical complement pathway. This not only helps to eliminate pathogens and dead cells, which is very useful in any case, but unfortunately also to remove only slightly damaged or inactive human cells that may potentially regenerate with more CRP-free time. CRP action severely aggravates the extent of tissue damage during the acute phase response after an acute injury and therefore negatively affects clinical outcome. CRP is therefore a promising therapeutic target to rescue energy-deprived tissue either caused by ischemic injury (e.g., myocardial infarction and stroke) or by an overcompensating immune reaction occurring in acute inflammation (e.g., pancreatitis) or systemic inflammatory response syndrome (SIRS; e.g., after transplantation or surgery). Selective CRP apheresis can remove circulating CRP safely and efficiently. We explain the pathophysiological reasoning behind therapeutic CRP apheresis and summarize the broad span of indications in which its application could be beneficial with a focus on ischemic stroke as well as the results of this therapeutic approach after myocardial infarction.
Keywords: CRP; apheresis; inflammation; stroke.
Conflict of interest statement
S.K. is an employee of Pentracor GmbH. J.D. received speaker honoraria and research fund from Fresenius Medical Care GmbH and Fresenius Medical Care Deutschland GmbH. A.S. is a founder, shareholder and managing director of Pentracor GmbH. P.B. and K.A. do not have any conflict of interest.
Figures
Similar articles
-
C-Reactive Protein Triggers Cell Death in Ischemic Cells.Front Immunol. 2021 Feb 10;12:630430. doi: 10.3389/fimmu.2021.630430. eCollection 2021. Front Immunol. 2021. PMID: 33679775 Free PMC article. Review.
-
[CRP apheresis in acute myocardial infarction and COVID-19].Med Klin Intensivmed Notfmed. 2022 Apr;117(3):191-199. doi: 10.1007/s00063-022-00911-x. Epub 2022 Mar 25. Med Klin Intensivmed Notfmed. 2022. PMID: 35333926 Free PMC article. Review. German.
-
Selective C-Reactive Protein-Apheresis in Patients.Ther Apher Dial. 2019 Dec;23(6):570-574. doi: 10.1111/1744-9987.12804. Epub 2019 Apr 29. Ther Apher Dial. 2019. PMID: 30924312
-
A Promising Innovative Treatment for ST-Elevation Myocardial Infarction: The Use of C-Reactive Protein Selective Apheresis: Case Report.Blood Purif. 2020;49(6):753-757. doi: 10.1159/000506176. Epub 2020 Feb 28. Blood Purif. 2020. PMID: 32114573
-
First-in-Man: Case Report of Selective C-Reactive Protein Apheresis in a Patient with SARS-CoV-2 Infection.Am J Case Rep. 2020 Jul 14;21:e925020. doi: 10.12659/AJCR.925020. Am J Case Rep. 2020. PMID: 32661220 Free PMC article.
Cited by
-
The role of apheresis and insulin therapy in hypertriglyceridemic acute pancreatitis-a concise review.BMC Gastroenterol. 2023 Oct 3;23(1):341. doi: 10.1186/s12876-023-02957-3. BMC Gastroenterol. 2023. PMID: 37789261 Free PMC article. Review.
-
Being Eaten Alive: How Energy-Deprived Cells Are Disposed of, Mediated by C-Reactive Protein-Including a Treatment Option.Biomedicines. 2023 Aug 16;11(8):2279. doi: 10.3390/biomedicines11082279. Biomedicines. 2023. PMID: 37626775 Free PMC article.
-
C-Reactive Protein Triggers Cell Death in Ischemic Cells.Front Immunol. 2021 Feb 10;12:630430. doi: 10.3389/fimmu.2021.630430. eCollection 2021. Front Immunol. 2021. PMID: 33679775 Free PMC article. Review.
-
Targeting C-Reactive Protein by Selective Apheresis in Humans: Pros and Cons.J Clin Med. 2022 Mar 23;11(7):1771. doi: 10.3390/jcm11071771. J Clin Med. 2022. PMID: 35407379 Free PMC article. Review.
-
Case Report: C-Reactive Protein Apheresis in a Patient With COVID-19 and Fulminant CRP Increase.Front Immunol. 2021 Aug 2;12:708101. doi: 10.3389/fimmu.2021.708101. eCollection 2021. Front Immunol. 2021. PMID: 34408751 Free PMC article.
References
-
- Ong S.-B., Hernández-Reséndiz S., Crespo-Avilan G.E., Mukhametshina R.T., Kwek X.-Y., Cabrera-Fuentes H.A., Hausenloy D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

