Organotypic Culture of Acinar Cells for the Study of Pancreatic Cancer Initiation
- PMID: 32932616
- PMCID: PMC7564199
- DOI: 10.3390/cancers12092606
Organotypic Culture of Acinar Cells for the Study of Pancreatic Cancer Initiation
Abstract
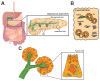
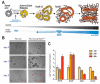
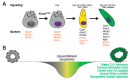
The carcinogenesis of pancreatic ductal adenocarcinoma (PDA) progresses according to multi-step evolution, whereby the disease acquires increasingly aggressive pathological features. On the other hand, disease inception is poorly investigated. Decoding the cascade of events that leads to oncogenic transformation is crucial to design strategies for early diagnosis as well as to tackle tumor onset. Lineage-tracing experiments demonstrated that pancreatic cancerous lesions originate from acinar cells, a highly specialized cell type in the pancreatic epithelium. Primary acinar cells can survive in vitro as organoid-like 3D spheroids, which can transdifferentiate into cells with a clear ductal morphology in response to different cell- and non-cell-autonomous stimuli. This event, termed acinar-to-ductal metaplasia, recapitulates the histological and molecular features of disease initiation. Here, we will discuss the isolation and culture of primary pancreatic acinar cells, providing a historical and technical perspective. The impact of pancreatic cancer research will also be debated. In particular, we will dissect the roles of transcriptional, epigenetic, and metabolic reprogramming for tumor initiation and we will show how that can be modeled using ex vivo acinar cell cultures. Finally, mechanisms of PDA initiation described using organotypical cultures will be reviewed.
Keywords: KRAS; acinar-to-ductal metaplasia; epigenetic reprogramming; metabolic reprogramming; organotypic culture; pancreas; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia.Oncogene. 2013 Apr 11;32(15):1950-8. doi: 10.1038/onc.2012.210. Epub 2012 Jun 4. Oncogene. 2013. PMID: 22665051 Free PMC article.
-
miR-802 Suppresses Acinar-to-Ductal Reprogramming During Early Pancreatitis and Pancreatic Carcinogenesis.Gastroenterology. 2022 Jan;162(1):269-284. doi: 10.1053/j.gastro.2021.09.029. Epub 2021 Sep 20. Gastroenterology. 2022. PMID: 34547282
-
GRP78 haploinsufficiency suppresses acinar-to-ductal metaplasia, signaling, and mutant Kras-driven pancreatic tumorigenesis in mice.Proc Natl Acad Sci U S A. 2017 May 16;114(20):E4020-E4029. doi: 10.1073/pnas.1616060114. Epub 2017 May 1. Proc Natl Acad Sci U S A. 2017. PMID: 28461470 Free PMC article.
-
Acinar to ductal cell trans-differentiation: A prelude to dysplasia and pancreatic ductal adenocarcinoma.Biochim Biophys Acta Rev Cancer. 2022 Jan;1877(1):188669. doi: 10.1016/j.bbcan.2021.188669. Epub 2021 Dec 13. Biochim Biophys Acta Rev Cancer. 2022. PMID: 34915061 Review.
-
Therapeutic potential of targeting acinar cell reprogramming in pancreatic cancer.World J Gastroenterol. 2016 Aug 21;22(31):7046-57. doi: 10.3748/wjg.v22.i31.7046. World J Gastroenterol. 2016. PMID: 27610015 Free PMC article. Review.
Cited by
-
Transcriptional Profile of Human Pancreatic Acinar Ductal Metaplasia.Gastro Hep Adv. 2023;2(4):532-543. doi: 10.1016/j.gastha.2023.02.003. Epub 2023 Feb 8. Gastro Hep Adv. 2023. PMID: 37425649 Free PMC article.
-
Generation of Hydrogen Peroxide and Downstream Protein Kinase D1 Signaling Is a Common Feature of Inducers of Pancreatic Acinar-to-Ductal Metaplasia.Antioxidants (Basel). 2022 Jan 8;11(1):137. doi: 10.3390/antiox11010137. Antioxidants (Basel). 2022. PMID: 35052641 Free PMC article.
-
Cerebellopontine angle metastasis from pancreatic cancer: a case report.Ann Med Surg (Lond). 2024 Sep 10;87(3):1652-1655. doi: 10.1097/MS9.0000000000002557. eCollection 2025 Mar. Ann Med Surg (Lond). 2024. PMID: 40213200 Free PMC article.
-
Cholesterol Activates Cyclic AMP Signaling in Metaplastic Acinar Cells.Metabolites. 2021 Feb 26;11(3):141. doi: 10.3390/metabo11030141. Metabolites. 2021. PMID: 33652890 Free PMC article.
-
The Role of Mitochondria in the Chemoresistance of Pancreatic Cancer Cells.Cells. 2021 Feb 25;10(3):497. doi: 10.3390/cells10030497. Cells. 2021. PMID: 33669111 Free PMC article. Review.
References
-
- Pourshams A., Sepanlou S.G., Ikuta K.S., Bisignano C., Safiri S., Roshandel G., Sharif M., Khatibian M., Fitzmaurice C., Nixon M.R., et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019;4:934–947. doi: 10.1016/S2468-1253(19)30347-4. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

