The Origin and Immune Recognition of Tumor-Specific Antigens
- PMID: 32932620
- PMCID: PMC7565792
- DOI: 10.3390/cancers12092607
The Origin and Immune Recognition of Tumor-Specific Antigens
Abstract
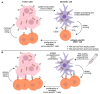
The dominant paradigm holds that spontaneous and therapeutically induced anti-tumor responses are mediated mainly by CD8 T cells and directed against tumor-specific antigens (TSAs). The presence of specific TSAs on cancer cells can only be proven by mass spectrometry analyses. Bioinformatic predictions and reverse immunology studies cannot provide this type of conclusive evidence. Most TSAs are coded by unmutated non-canonical transcripts that arise from cancer-specific epigenetic and splicing aberrations. When searching for TSAs, it is therefore important to perform mass spectrometry analyses that interrogate not only the canonical reading frame of annotated exome but all reading frames of the entire translatome. The majority of aberrantly expressed TSAs (aeTSAs) derive from unstable short-lived proteins that are good substrates for direct major histocompatibility complex (MHC) I presentation but poor substrates for cross-presentation. This is an important caveat, because cancer cells are poor antigen-presenting cells, and the immune system, therefore, depends on cross-presentation by dendritic cells (DCs) to detect the presence of TSAs. We, therefore, postulate that, in the untreated host, most aeTSAs are undetected by the immune system. We present evidence suggesting that vaccines inducing direct aeTSA presentation by DCs may represent an attractive strategy for cancer treatment.
Keywords: T lymphocyte; antigen processing and presentation; cancer immunotherapy; cross-priming; immunogenicity; major histocompatibility complex; tumor microenvironment; tumor-infiltrating lymphocytes; tumor-specific antigen.
Conflict of interest statement
M.P.H., P.T. and C.P. are named inventors on patents related to tumor-specific antigens filed by Université de Montréal. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

