Effects of Experimental Agents Containing Tannic Acid or Chitosan on the Bacterial Biofilm Formation in Situ
- PMID: 32932628
- PMCID: PMC7564313
- DOI: 10.3390/biom10091315
Effects of Experimental Agents Containing Tannic Acid or Chitosan on the Bacterial Biofilm Formation in Situ
Abstract
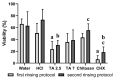
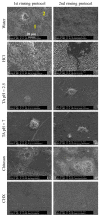
Chitosan and tannic acid are known for their antibacterial properties. In the present in-situ study, their antibacterial and anti-adherent effects on biofilm formation on enamel were investigated. Six subjects carried upper jaw splints with bovine enamel specimens, allowing in-situ biofilm formation. During the two-day trial, subjects rinsed with experimental solutions that contained either chitosan, tannic acid (pH = 2.5), tannic acid (pH = 7) or hydrochloric acid. Water served as the negative and chlorhexidine as the positive control. Rinsing occurred four or five times following two different rinsing protocols to investigate both the immediate and long-lasting effects. After 48 h of intraoral exposure, the dental plaque was stained with LIVE/DEAD® BacLight, and fluorescence micrographs were evaluated by using the software ImageJ. The results were verified by scanning electron microscopy. Rinsing with chitosan resulted in little immediate antibacterial and anti-adherent effects but failed to show any long-lasting effect, while rinsing with tannic acid resulted in strong immediate and long-lasting effects. Except for a slightly lower antibacterial effect, the neutral solution of tannic acid was as good as the acidic solution. Hydrochloric acid showed neither an antibacterial nor an anti-adherent effect on dental biofilm formation. Experimental solutions containing tannic acid are promising anti-biofilm agents, irrespective of the pH values of the solutions. Chitosan, on the other hand, was not able to prevent biofilm formation.
Keywords: biofilm; chitosan; tannic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kassebaum N., Smith A.G., Bernabé E., Fleming T., Reynolds A., Vos T., Murray C., Marcenes W., Abyu G. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017;96:380–387. doi: 10.1177/0022034517693566. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

