Optimized Identification of Advanced Chronic Kidney Disease and Absence of Kidney Disease by Combining Different Electronic Health Data Resources and by Applying Machine Learning Strategies
- PMID: 32932685
- PMCID: PMC7563476
- DOI: 10.3390/jcm9092955
Optimized Identification of Advanced Chronic Kidney Disease and Absence of Kidney Disease by Combining Different Electronic Health Data Resources and by Applying Machine Learning Strategies
Abstract
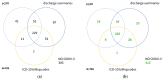
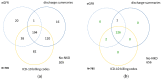
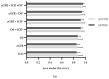
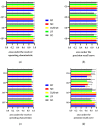
Automated identification of advanced chronic kidney disease (CKD ≥ III) and of no known kidney disease (NKD) can support both clinicians and researchers. We hypothesized that identification of CKD and NKD can be improved, by combining information from different electronic health record (EHR) resources, comprising laboratory values, discharge summaries and ICD-10 billing codes, compared to using each component alone. We included EHRs from 785 elderly multimorbid patients, hospitalized between 2010 and 2015, that were divided into a training and a test (n = 156) dataset. We used both the area under the receiver operating characteristic (AUROC) and under the precision-recall curve (AUCPR) with a 95% confidence interval for evaluation of different classification models. In the test dataset, the combination of EHR components as a simple classifier identified CKD ≥ III (AUROC 0.96[0.93-0.98]) and NKD (AUROC 0.94[0.91-0.97]) better than laboratory values (AUROC CKD 0.85[0.79-0.90], NKD 0.91[0.87-0.94]), discharge summaries (AUROC CKD 0.87[0.82-0.92], NKD 0.84[0.79-0.89]) or ICD-10 billing codes (AUROC CKD 0.85[0.80-0.91], NKD 0.77[0.72-0.83]) alone. Logistic regression and machine learning models improved recognition of CKD ≥ III compared to the simple classifier if only laboratory values were used (AUROC 0.96[0.92-0.99] vs. 0.86[0.81-0.91], p < 0.05) and improved recognition of NKD if information from previous hospital stays was used (AUROC 0.99[0.98-1.00] vs. 0.95[0.92-0.97]], p < 0.05). Depending on the availability of data, correct automated identification of CKD ≥ III and NKD from EHRs can be improved by generating classification models based on the combination of different EHR components.
Keywords: ICD-10 billing codes; area under the precision-recall curve (AUCPR); area under the receiver operating characteristic (AUROC); artificial neural network (ANN), clinical natural language processing (clinical NLP); chronic kidney disease (CKD); discharge summaries; electronic health record (EHR); estimated glomerular filtration rate (eGFR); generalized linear model network (GLMnet); laboratory values; machine learning (ML); no known kidney disease (NKD); phenotyping; random forest (RF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Xie Y., Bowe B., Mokdad A.H., Xian H., Yan Y., Li T., Maddukuri G., Tsai C.Y., Floyd T., Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. - DOI - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013;3:1–150.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

