Cutibacterium acnes Biofilm Study during Bone Cells Interaction
- PMID: 32932750
- PMCID: PMC7564252
- DOI: 10.3390/microorganisms8091409
Cutibacterium acnes Biofilm Study during Bone Cells Interaction
Abstract
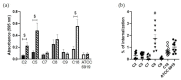
Cutibacterium acnes is an opportunistic pathogen involved in Bone and Prosthesis Infections (BPIs). In this study, we observed the behavior of commensal and BPI C. acnes strains in the bone environment through bacterial internalization by osteoblast-like cells and biofilm formation. For the commensal strains, less than 1% of the bacteria were internalized; among them, about 32.7 ± 3.9% persisted intracellularly for up to 48 h. C. acnes infection seems to have no cytotoxic effect on bone cells as detected by LDH assay. Interestingly, commensal C. acnes showed a significant increase in biofilm formation after osteoblast-like internalization for 50% of the strains (2.8-fold increase). This phenomenon is exacerbated on a titanium support, a material used for medical devices. For the BPI clinical strains, we did not notice any increase in biofilm formation after internalization despite a similar internalization rate by the osteoblast-like cells. Furthermore, fluorescent staining revealed more live bacteria within the biofilm after osteoblast-like cell interaction, for all strains (BPIs and commensal). The genomic study did not reveal any link between their clinical origin and phylotype. In conclusion, we have shown for the first time the possible influence of internalization by osteoblast-like cells on commensal C. acnes.
Keywords: Cutibacterium acnes; biofilms; host–pathogen interactions; internalization; joint infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Anagnostopoulos A., Bossard D.A., Ledergerber B., Zingg P.O., Zinkernagel A.S., Gerber C., Achermann Y. Perioperative Antibiotic Prophylaxis Has No Effect on Time to Positivity and Proportion of Positive Samples: A Cohort Study of 64 Cutibacterium acnes Bone and Joint Infections. J Clin. Microbiol. 2018;56:e01576-17. doi: 10.1128/JCM.01576-17. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

