Intensive Running Enhances NF-κB Activity in the Mice Liver and the Intervention Effects of Quercetin
- PMID: 32932805
- PMCID: PMC7551556
- DOI: 10.3390/nu12092770
Intensive Running Enhances NF-κB Activity in the Mice Liver and the Intervention Effects of Quercetin
Abstract
Background: Emerging evidence has supported that intensive exercise induces weakened performance and immune and metabolic disorders. We systematically evaluated the effects of quercetin against hepatic inflammatory damage caused by repeated intensive exercise and explored the potential mechanism.
Methods: Male BALB/c mice were administered quercetin (100 mg/kg BW) for four weeks, and performed a treadmill running protocol of 28 m/min, 5° slope, 90 min/day concurrently for the last seven days.
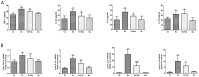
Results: Quercetin administration reduced the leakage of aspartic acid and alanine aminotransferase and improved ultrastructural abnormalities such as swelling, and degeneration caused by high-intensity running in mice. Quercetin significantly decreased the hepatic and plasmatic levels of inflammatory cytokines IL-1β, IL-6, TNF-α, inducible nitric oxide synthase, cyclooxygenase-2 and intercellular adhesion molecule-1-provoked by over-exercise. Furthermore, diminished activation and nuclear translocation of NF-κB were found after quercetin treatment through inhibiting IKKα and Iκbα phosphorylation of intensive running mice.
Conclusion: Quercetin offers protection for mouse livers against intensive sports-induced inflammatory injury, and the suppression of the NF-κB signal transduction pathway may play a role in its anti-inflammatory effects. Our findings broaden our understanding of natural phytochemicals as a promising strategy to prevent excessive exercise damage.
Keywords: inflammation; intensive exercise; liver damage; quercetin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

