Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells
- PMID: 32932813
- PMCID: PMC7565696
- DOI: 10.3390/cells9092076
Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells
Abstract
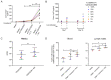
The accurate estimation and eradication of Human Immunodeficiency Virus (HIV) viral reservoirs is limited by the incomplete reactivation of cells harboring the latent replication-competent virus. We investigated whether the in vitro and in vivo addition of retinoic acid (RA) enhances virus replication and improves the detection of latent virus. Peripheral blood mononuclear cells (PBMCs) from naive and anti-retroviral therapy (ART)-treated SIV-infected rhesus macaques (RMs) were cultured in vitro with anti-CD3/CD28 + IL-2 in the presence/absence of RA. Viral RNA and p27 levels were quantified using RT-qPCR and ELISA, respectively. Viral reservoirs were estimated using the Tat/Rev-Induced Limited Dilution Assay (TILDA) and Quantitative Viral Outgrowth Assay (QVOA). In vitro and in vivo measures revealed that there was also an increase in viral replication in RA-treated versus without RA conditions. In parallel, the addition of RA to either CD3/CD28 or phorbol myristate acetate (PMA)/ionomycin during QVOA and TILDA, respectively, was shown to augment reactivation of the replication-competent viral reservoir in anti-retroviral therapy (ART)-suppressed RMs as shown by a greater than 2.3-fold increase for QVOA and 1 to 2-fold increments for multi-spliced RNA per million CD4+ T cells. The use of RA can be a useful approach to enhance the efficiency of current protocols used for in vitro and potentially in vivo estimates of CD4+ T cell latent reservoirs. In addition, flow cytometry analysis revealed that RA improved estimates of various viral reservoir assays by eliciting broad CD4 T-cell activation as demonstrated by elevated CD25 and CD38 but reduced CD69 and PD-1 expressing cells.
Keywords: CD3/CD28 treatment; CD4 T cells; PBMCs; SIV; immune activation; retinoic acid; viral load and provirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cohen C. Second ‘Cured’ HIV Patient Goes Public. [(accessed on 25 June 2020)]; Available online: https://www.sciencemag.org/news/2020/03/second-cured-hiv-patient-goes-pu....
-
- Henrich T.J., Hanhauser E., Marty F.M., Sirignano M.N., Keating S., Lee T.H., Robles Y.P., Davis B.T., Li J.Z., Heisey A., et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: Report of 2 cases. Ann. Intern. Med. 2014;161:319–327. doi: 10.7326/M14-1027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

