Analyzing the Potential Biological Determinants of Autism Spectrum Disorder: From Neuroinflammation to the Kynurenine Pathway
- PMID: 32932826
- PMCID: PMC7563403
- DOI: 10.3390/brainsci10090631
Analyzing the Potential Biological Determinants of Autism Spectrum Disorder: From Neuroinflammation to the Kynurenine Pathway
Abstract
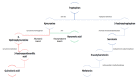
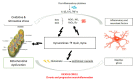
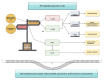
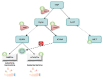
Autism Spectrum Disorder (ASD) etiopathogenesis is still unclear and no effective preventive and treatment measures have been identified. Research has focused on the potential role of neuroinflammation and the Kynurenine pathway; here we review the nature of these interactions. Pre-natal or neonatal infections would induce microglial activation, with secondary consequences on behavior, cognition and neurotransmitter networks. Peripherally, higher levels of pro-inflammatory cytokines and anti-brain antibodies have been identified. Increased frequency of autoimmune diseases, allergies, and recurring infections have been demonstrated both in autistic patients and in their relatives. Genetic studies have also identified some important polymorphisms in chromosome loci related to the human leukocyte antigen (HLA) system. The persistence of immune-inflammatory deregulation would lead to mitochondrial dysfunction and oxidative stress, creating a self-sustaining cytotoxic loop. Chronic inflammation activates the Kynurenine pathway with an increase in neurotoxic metabolites and excitotoxicity, causing long-term changes in the glutamatergic system, trophic support and synaptic function. Furthermore, overactivation of the Kynurenine branch induces depletion of melatonin and serotonin, worsening ASD symptoms. Thus, in genetically predisposed subjects, aberrant neurodevelopment may derive from a complex interplay between inflammatory processes, mitochondrial dysfunction, oxidative stress and Kynurenine pathway overexpression. To validate this hypothesis a new translational research approach is necessary.
Keywords: KYNA (kynurenic acid); Kynurenine pathway; QUIN (quinolinic acid); autism spectrum disorder; immune deregulation; microglia; mitochondrial disorder; neuroinflammation; oxidative stress; tryptophan catabolites.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Washington, DC, USA: 2013.
-
- Baio J., Wiggins L., Christensen D.L., Maenner M.J., Daniels J., Warren Z., Kurzius-Spencer M., Zahorodny W., Robinson Rosenberg C., White T., et al. Prevalence of Autism Spectrum Disorder among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018;67:1–23. doi: 10.15585/mmwr.ss6706a1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials

