Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape
- PMID: 32932957
- PMCID: PMC7559083
- DOI: 10.3390/nano10091816
Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape
Abstract
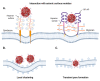
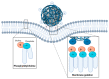
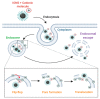
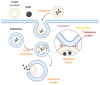
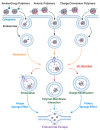
Iron oxide nanoparticles (IONs) have been widely explored for biomedical applications due to their high biocompatibility, surface-coating versatility, and superparamagnetic properties. Upon exposure to an external magnetic field, IONs can be precisely directed to a region of interest and serve as exceptional delivery vehicles and cellular markers. However, the design of nanocarriers that achieve an efficient endocytic uptake, escape lysosomal degradation, and perform precise intracellular functions is still a challenge for their application in translational medicine. This review highlights several aspects that mediate the activation of the endosomal pathways, as well as the different properties that govern endosomal escape and nuclear transfection of magnetic IONs. In particular, we review a variety of ION surface modification alternatives that have emerged for facilitating their endocytic uptake and their timely escape from endosomes, with special emphasis on how these can be manipulated for the rational design of cell-penetrating vehicles. Moreover, additional modifications for enhancing nuclear transfection are also included in the design of therapeutic vehicles that must overcome this barrier. Understanding these mechanisms opens new perspectives in the strategic development of vehicles for cell tracking, cell imaging and the targeted intracellular delivery of drugs and gene therapy sequences and vectors.
Keywords: drug delivery; endocytosis; endosomal escape; iron oxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Zoppellaro G. Magnetic Nanoheterostructures. Springer International Publishing; Cham, Switzerland: 2020. Iron Oxide Magnetic Nanoparticles (NPs) Tailored for Biomedical Applications; pp. 57–102. - DOI
-
- Bohara R.A., Singh P. Magnetic Nanoheterostructures. Springer International Publishing; Cham, Switzerland: 2020. Multiple Myeloma: Role of Magnetic Nanoparticles; pp. 479–494. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

