Levetiracetam Reduced the Basal Excitability of the Dentate Gyrus without Restoring Impaired Synaptic Plasticity in Rats with Temporal Lobe Epilepsy
- PMID: 32933015
- PMCID: PMC7565946
- DOI: 10.3390/brainsci10090634
Levetiracetam Reduced the Basal Excitability of the Dentate Gyrus without Restoring Impaired Synaptic Plasticity in Rats with Temporal Lobe Epilepsy
Abstract
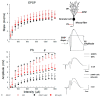
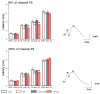
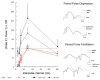
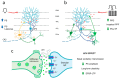
Temporal lobe epilepsy (TLE), the most common type of focal epilepsy, affects learning and memory; these effects are thought to emerge from changes in synaptic plasticity. Levetiracetam (LEV) is a widely used antiepileptic drug that is also associated with the reversal of cognitive dysfunction. The long-lasting effect of LEV treatment and its participation in synaptic plasticity have not been explored in early chronic epilepsy. Therefore, through the measurement of evoked field potentials, this study aimed to comprehensively identify the alterations in the excitability and the short-term (depression/facilitation) and long-term synaptic plasticity (long-term potentiation, LTP) of the dentate gyrus of the hippocampus in a lithium-pilocarpine rat model of TLE, as well as their possible restoration by LEV (1 week; 300 mg/kg/day). TLE increased the population spike (PS) amplitude (input/output curve); interestingly, LEV treatment partially reduced this hyperexcitability. Furthermore, TLE augmented synaptic depression, suppressed paired-pulse facilitation, and reduced PS-LTP; however, LEV did not alleviate such alterations. Conversely, the excitatory postsynaptic potential (EPSP)-LTP of TLE rats was comparable to that of control rats and was decreased by LEV. LEV caused a long-lasting attenuation of basal hyperexcitability but did not restore impaired synaptic plasticity in the early chronic phase of TLE.
Keywords: evoked field potentials; inhibitory transmission; levetiracetam; synaptic plasticity; temporal lobe epilepsy.
Conflict of interest statement
The authors declare no conflicts of interest, financial or otherwise.
Figures




References
-
- Stafstrom C.E. Pathophysiological mechanisms of seizures and epilepsy: A primer. In: Rho J.M., Sankar R., Stafstrom C.E., editors. Epilepsy: Mechanisms, Models, and Translational Perspectives. CRC Press; Boca Raton, FL, USA: 2010. pp. 3–17.
Grants and funding
LinkOut - more resources
Full Text Sources

