Ionocytes and CFTR Chloride Channel Expression in Normal and Cystic Fibrosis Nasal and Bronchial Epithelial Cells
- PMID: 32933106
- PMCID: PMC7565890
- DOI: 10.3390/cells9092090
Ionocytes and CFTR Chloride Channel Expression in Normal and Cystic Fibrosis Nasal and Bronchial Epithelial Cells
Abstract
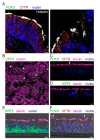
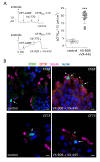
The airway epithelium contains ionocytes, a rare cell type with high expression of Forkhead Box I1 (FOXI1) transcription factor and Cystic Fibrosis Transmembrane conductance Regulator (CFTR), a chloride channel that is defective in cystic fibrosis (CF). Our aim was to verify if ionocyte development is altered in CF and to investigate the relationship between ionocytes and CFTR-dependent chloride secretion. We collected nasal cells by brushing to determine ionocyte abundance. Nasal and bronchial cells were also expanded in vitro and reprogrammed to differentiated epithelia for morphological and functional studies. We found a relatively high (~3%) ionocyte abundance in ex vivo nasal samples, with no difference between CF and control individuals. In bronchi, ionocytes instead appeared very rarely as previously reported, thus suggesting a possible proximal-distal gradient in human airways. The difference between nasal and bronchial epithelial cells was maintained in culture, which suggests an epigenetic control of ionocyte development. In the differentiation phase of the culture procedure, we used two media that resulted in a different pattern of CFTR expression: confined to ionocytes or more broadly expressed. CFTR function was similar in both conditions, thus indicating that chloride secretion equally occurs irrespective of CFTR expression pattern.
Keywords: CFTR; airway epithelium; chloride secretion; cystic fibrosis; ionocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Scudieri P., Caci E., Bruno S., Ferrera L., Schiavon M., Sondo E., Tomati V., Gianotti A., Zegarra-Moran O., Pedemonte N., et al. Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J. Physiol. 2012;590:6141–6155. doi: 10.1113/jphysiol.2012.240838. - DOI - PMC - PubMed

