Manufacture of a Stable Lyophilized Formulation of the Live Attenuated Pertussis Vaccine BPZE1
- PMID: 32933132
- PMCID: PMC7565209
- DOI: 10.3390/vaccines8030523
Manufacture of a Stable Lyophilized Formulation of the Live Attenuated Pertussis Vaccine BPZE1
Abstract
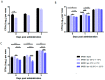
Current pertussis vaccines protect against disease, but not against colonization by and transmission of Bordetella pertussis, whereas natural infection protects against both. The live attenuated vaccine BPZE1 was developed to mimic immunogenicity of natural infection without causing disease, and in preclinical models protected against pertussis disease and B. pertussis colonization after a single nasal administration. Phase 1 clinical studies showed that BPZE1 is safe and immunogenic in humans when administered as a liquid formulation, stored at ≤-70 °C. Although BPZE1 is stable for two years at ≤-70 °C, a lyophilized formulation stored at ≥5 °C is required for commercialization. The development of a BPZE1 drug product, filled and lyophilized directly in vials, showed that post-lyophilization survival of BPZE1 depended on the time of harvest, the lyophilization buffer, the time between harvest and lyophilization, as well as the lyophilization cycle. The animal component-free process, well defined in terms of harvest, processing and lyophilization, resulted in approximately 20% survival post-lyophilization. The resulting lyophilized drug product was stable for at least two years at -20 °C ± 10 °C, 5 °C ± 3 °C and 22.5 °C ± 2.5 °C and maintained its vaccine potency, as evaluated in a murine protection assay. This manufacturing process thus enables further clinical and commercial development of BPZE1.
Keywords: live attenuated vaccine; lyophilization; pertussis; vaccine stability.
Conflict of interest statement
M.T., K.S. and K.R. are employed by ILiAD Biotechnologies; M.T. is employed by BioLyo Technologies; A.-S.D., D.R., C.L. and N.M. hold patents on the BPZE1 vaccine, which are licensed to ILiAD Biotechnologies. All other authors declare that they have no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

