In Vitro Selection of an ATP-Binding TNA Aptamer
- PMID: 32933142
- PMCID: PMC7570665
- DOI: 10.3390/molecules25184194
In Vitro Selection of an ATP-Binding TNA Aptamer
Abstract
Recent advances in polymerase engineering have made it possible to isolate aptamers from libraries of synthetic genetic polymers (XNAs) with backbone structures that are distinct from those found in nature. However, nearly all of the XNA aptamers produced thus far have been generated against protein targets, raising significant questions about the ability of XNA aptamers to recognize small molecule targets. Here, we report the evolution of an ATP-binding aptamer composed entirely of α-L-threose nucleic acid (TNA). A chemically synthesized version of the best aptamer sequence shows high affinity to ATP and strong specificity against other naturally occurring ribonucleotide triphosphates. Unlike its DNA and RNA counterparts that are susceptible to nuclease digestion, the ATP-binding TNA aptamer exhibits high biological stability against hydrolytic enzymes that rapidly degrade DNA and RNA. Based on these findings, we suggest that TNA aptamers could find widespread use as molecular recognition elements in diagnostic and therapeutic applications that require high biological stability.
Keywords: TNA; aptamer; biological stability.
Conflict of interest statement
We have no competing interests.
Figures
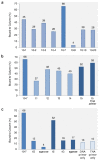
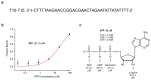

References
-
- Dunn M.R., Jimenez R.M., Chaput J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017;1:0076. doi: 10.1038/s41570-017-0076. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

