Host Synthesized Carbohydrate Antigens on Viral Glycoproteins as "Achilles' Heel" of Viruses Contributing to Anti-Viral Immune Protection
- PMID: 32933166
- PMCID: PMC7555091
- DOI: 10.3390/ijms21186702
Host Synthesized Carbohydrate Antigens on Viral Glycoproteins as "Achilles' Heel" of Viruses Contributing to Anti-Viral Immune Protection
Abstract
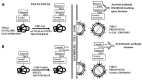
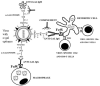
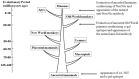
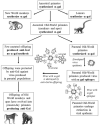
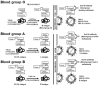
The glycans on enveloped viruses are synthesized by host-cell machinery. Some of these glycans on zoonotic viruses of mammalian reservoirs are recognized by human natural antibodies that may protect against such viruses. These antibodies are produced mostly against carbohydrate antigens on gastrointestinal bacteria and fortuitously, they bind to carbohydrate antigens synthesized in other mammals, neutralize and destroy viruses presenting these antigens. Two such antibodies are: anti-Gal binding to α-gal epitopes synthesized in non-primate mammals, lemurs, and New World monkeys, and anti-N-glycolyl neuraminic acid (anti-Neu5Gc) binding to N-glycolyl-neuraminic acid (Neu5Gc) synthesized in apes, Old World monkeys, and many non-primate mammals. Anti-Gal appeared in Old World primates following accidental inactivation of the α1,3galactosyltransferase gene 20-30 million years ago. Anti-Neu5Gc appeared in hominins following the inactivation of the cytidine-monophosphate-N-acetyl-neuraminic acid hydroxylase gene, which led to the loss of Neu5Gc <6 million-years-ago. It is suggested that an epidemic of a lethal virus eliminated ancestral Old World-primates synthesizing α-gal epitopes, whereas few mutated offspring lacking α-gal epitopes and producing anti-Gal survived because anti-Gal destroyed viruses presenting α-gal epitopes, following replication in parental populations. Similarly, anti-Neu5Gc protected few mutated hominins lacking Neu5Gc in lethal virus epidemics that eliminated parental hominins synthesizing Neu5Gc. Since α-gal epitopes are presented on many zoonotic viruses it is suggested that vaccines elevating anti-Gal titers may be of protective significance in areas endemic for such zoonotic viruses. This protection would be during the non-primate mammal to human virus transmission, but not in subsequent human to human transmission where the virus presents human glycans. In addition, production of viral vaccines presenting multiple α-gal epitopes increases their immunogenicity because of effective anti-Gal-mediated targeting of vaccines to antigen presenting cells for extensive uptake of the vaccine by these cells.
Keywords: Covid-19; Neu5Gc; anti-Gal; anti-Neu5Gc; blood groups ABO and Bombay; glycan shield; viral epidemics; viral vaccines; zoonotic virus; α-gal epitopes.
Conflict of interest statement
The author is the inventor in US patents 9662383 and 10201601 (Assignee, University of Massachusetts), which include some of the methods described in this review.
Figures
References
-
- Xiong X., Tortorici M.A., Snijder J., Yoshioka C., Walls A.C., Li W., McGuire A.T., Rey F.A., Bosch B.J., Veesler D. Glycan Shield and Fusion Activation of a Deltacoronavirus Spike Glycoprotein Fine-Tuned for Enteric Infections. J. Virol. 2018;92:e01628-17. doi: 10.1128/JVI.01628-17. - DOI - PMC - PubMed
-
- Crooks E., Tong T., Chakrabarti B., Narayan K., Georgiev I., Menis S. Vaccine-elicited tier 2 HIV-1 neutralizing antibodies bind to quaternary epitopes involving glycan-deficient patches Proximal to the CD4 Binding Site. PLoS Pathog. 2015;11:e1004932. doi: 10.1371/journal.ppat.1004932. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

