Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function
- PMID: 32933181
- PMCID: PMC7551690
- DOI: 10.3390/nu12092808
Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function
Abstract
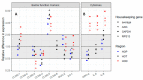
Human milk oligosaccharides (HMOs) shape the gut microbiota in infants by selectively stimulating the growth of bifidobacteria. Here, we investigated the impact of HMOs on adult gut microbiota and gut barrier function using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®), Caco2 cell lines, and human intestinal gut organoid-on-chips. We showed that fermentation of 2'-O-fucosyllactose (2'FL), lacto-N-neotetraose (LNnT), and combinations thereof (MIX) led to an increase of bifidobacteria, accompanied by an increase of short chain fatty acid (SCFA), in particular butyrate with 2'FL. A significant reduction in paracellular permeability of FITC-dextran probe was observed using Caco2 cell monolayers with fermented 2'FL and MIX, which was accompanied by an increase in claudin-8 gene expression as shown by qPCR, and a reduction in IL-6 as determined by multiplex ELISA. Using gut-on-chips generated from human organoids derived from proximal, transverse, and distal colon biopsies (Colon Intestine Chips), we showed that claudin-5 was significantly upregulated across all three gut-on-chips following treatment with fermented 2'FL under microfluidic conditions. Taken together, these data show that, in addition to their bifidogenic activity, HMOs have the capacity to modulate immune function and the gut barrier, supporting the potential of HMOs to provide health benefits in adults.
Keywords: SHIME®; adult gut microbiota; gut barrier function; gut-on-chips; human milk oligosaccharides.
Conflict of interest statement
The authors declare no conflict of interest. L.K.V. and B.M.C. participated in the design of the study, the interpretation of the data, and the writing of the manuscript but did not participate in the collection and analyses of data and encouraged publication of the study.
Figures





References
-
- Cabrera-Rubio R., Kunz C., Rudloff S., García-Mantrana I., Crehuá-Gaudiza E., Martínez-Costa C., Collado M.C. Association of Maternal Secretor Status and Human Milk Oligosaccharides with Milk Microbiota: An Observational Pilot Study. J. Pediatr. Gastroenterol. Nutr. 2019;68:256–263. doi: 10.1097/MPG.0000000000002216. - DOI - PubMed
-
- Bode L. Human Milk Oligosaccharides: Next-Generation Functions and Questions. Nestle Nutr. Inst. Workshop Ser. 2019;90:191–201. - PubMed
MeSH terms
Substances
Grants and funding
- BBS/E/F/00044452/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10356/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/CCG1860/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

