Doubly-Charged Negative Ions as Novel Tunable Catalysts: Graphene and Fullerene Molecules Versus Atomic Metals
- PMID: 32933219
- PMCID: PMC7554846
- DOI: 10.3390/ijms21186714
Doubly-Charged Negative Ions as Novel Tunable Catalysts: Graphene and Fullerene Molecules Versus Atomic Metals
Abstract
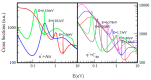
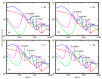
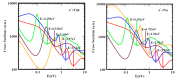
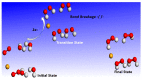
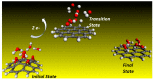
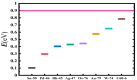
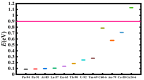
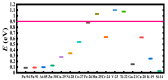
The fundamental mechanism underlying negative-ion catalysis involves bond-strength breaking in the transition state (TS). Doubly-charged atomic/molecular anions are proposed as novel dynamic tunable catalysts, as demonstrated in water oxidation into peroxide. Density Functional Theory TS calculations have found a tunable energy activation barrier reduction ranging from 0.030 eV to 2.070 eV, with Si2-, Pu2-, Pa2- and Sn2- being the best catalysts; the radioactive elements usher in new application opportunities. C602- significantly reduces the standard C60- TS energy barrier, while graphene increases it, behaving like cationic systems. According to their reaction barrier reduction efficiency, variation across charge states and systems, rank-ordered catalysts reveal their tunable and wide applications, ranging from water purification to biocompatible antiviral and antibacterial sanitation systems.
Keywords: Graphene; atomic metals; doubly-charged anions; electron scattering; fullerenes; tunable catalysts; water oxidation.
Conflict of interest statement
The authors declare no conflict of interest or state.
Figures


References
-
- Sartorel A., Carraro M., Scorrano G., Bonchio M. Water oxidation catalysis by molecular metal-oxides. Energy Procedia. 2012;22:78–87. doi: 10.1016/j.egypro.2012.05.228. - DOI
-
- Du P., Eisenberg R. Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: Recent progress and future challenges. Energy Environ. Sci. 2012;5:6012. doi: 10.1039/c2ee03250c. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

