Transmembrane potential of physiologically relevant model membranes: Effects of membrane asymmetry
- PMID: 32933265
- PMCID: PMC7484991
- DOI: 10.1063/5.0018303
Transmembrane potential of physiologically relevant model membranes: Effects of membrane asymmetry
Abstract
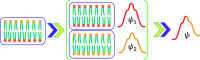
Transmembrane potential difference (Vm) plays important roles in regulating various biological processes. At the macro level, Vm can be experimentally measured or calculated using the Nernst or Goldman-Hodgkin-Katz equation. However, the atomic details responsible for its generation and impact on protein and lipid dynamics still need to be further elucidated. In this work, we performed a series of all-atom molecular dynamics (MD) simulations of symmetric model membranes of various lipid compositions and cation contents to evaluate the relationship between membrane asymmetry and Vm. Specifically, we studied the impact of the asymmetric distribution of POPS (1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine), PIP2 (phosphatidylinositol 4,5-bisphosphate), as well as Na+ and K+ on Vm using atomically detailed MD simulations of symmetric model membranes. The results suggest that, for an asymmetric POPC-POPC/POPS bilayer in the presence of NaCl, the presence of the monovalent anionic lipid POPS in the inner leaflet polarizes the membrane (ΔVm < 0). Intriguingly, replacing a third of the POPS lipids by the polyvalent anionic signaling lipid PIP2 counteracts this effect, resulting in a smaller negative membrane potential. We also found that replacing Na+ ions in the inner region by K+ depolarizes the membrane (ΔVm > 0). These divergent effects arise from variations in the strength of cation-lipid interactions and are correlated with changes in lipid chain order and head-group orientation.
Figures



Similar articles
-
Penetratin translocation mechanism through asymmetric droplet interface bilayers.Biochim Biophys Acta Biomembr. 2020 Nov 1;1862(11):183415. doi: 10.1016/j.bbamem.2020.183415. Epub 2020 Jul 22. Biochim Biophys Acta Biomembr. 2020. PMID: 32710854
-
Interaction of Human β Defensin Type 3 (hBD-3) with Different PIP2-Containing Membranes, a Molecular Dynamics Simulation Study.J Chem Inf Model. 2021 Sep 27;61(9):4670-4686. doi: 10.1021/acs.jcim.1c00805. Epub 2021 Sep 2. J Chem Inf Model. 2021. PMID: 34473496
-
A Coarse Grained Model for a Lipid Membrane with Physiological Composition and Leaflet Asymmetry.PLoS One. 2015 Dec 14;10(12):e0144814. doi: 10.1371/journal.pone.0144814. eCollection 2015. PLoS One. 2015. PMID: 26659855 Free PMC article.
-
Structure, dynamics, and hydration of POPC/POPS bilayers suspended in NaCl, KCl, and CsCl solutions.Biochim Biophys Acta. 2012 Mar;1818(3):609-16. doi: 10.1016/j.bbamem.2011.11.033. Epub 2011 Dec 6. Biochim Biophys Acta. 2012. PMID: 22155683
-
Building Asymmetric Lipid Bilayers for Molecular Dynamics Simulations: What Methods Exist and How to Choose One?Membranes (Basel). 2023 Jun 29;13(7):629. doi: 10.3390/membranes13070629. Membranes (Basel). 2023. PMID: 37504995 Free PMC article. Review.
References
-
- Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., and Walter P., Molecular Biology of the Cell, 6th ed. (Garland Press, New York, 2014).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

