Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: Role of elevated 2,3-biphosphoglycerate in erythrocytes
- PMID: 32933360
- PMCID: PMC8142126
- DOI: 10.1177/0271678X20952264
Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: Role of elevated 2,3-biphosphoglycerate in erythrocytes
Abstract
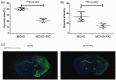
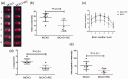
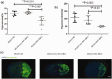
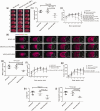
Oxygen supply for ischemic brain tissue during stroke is critical to neuroprotection. Remote ischemic conditioning (RIC) treatment is effective for stroke. However, it is not known whether RIC can improve brain tissue oxygen supply. In current study, we employed a mouse model of stroke created by middle cerebral artery occlusion (MCAO) to investigate the effect of RIC on oxygen supply to the ischemic brain tissue using a hypoxyprobe system. Erythrocyte oxygen-carrying capacity and tissue oxygen exchange were assessed by measuring oxygenated hemoglobin and oxygen dissociation curve. We found that RIC significantly mitigated hypoxic signals and decreased neural cell death, thereby preserving neurological functions. The tissue oxygen exchange was markedly enhanced, along with the elevated hemoglobin P50 and right-shifted oxygen dissociation curve. Intriguingly, RIC markedly elevated 2,3-biphosphoglycerate (2,3-BPG) levels in erythrocyte, and the erythrocyte 2,3-BPG levels were highly negatively correlated with the hypoxia in the ischemic brain tissue. Further, adoptive transfusion of 2,3-BPG-rich erythrocytes prepared from RIC-treated mice significantly enhanced the oxygen supply to the ischemic tissue in MCAO mouse model. Collectively, RIC protects against ischemic stroke through improving oxygen supply to the ischemic brain tissue where the enhanced tissue oxygen delivery and exchange by RIC-induced 2,3-BPG-rich erythrocytes may play a role.
Keywords: 2,3-biphosphoglycerate/2,3-diphosphoglycerate; Remote ischemic conditioning; hypoxia; oxygen dissociation curve; stroke.
Conflict of interest statement
Figures


Similar articles
-
Comparative Efficacy of Remote Ischemic Conditioning and Hypothermia in Permanent and Transient Cerebral Ischemia in Male Mice.J Neurosci Res. 2024 Dec;102(12):e70003. doi: 10.1002/jnr.70003. J Neurosci Res. 2024. PMID: 39722429
-
Remote Limb Ischemic Conditioning during Cerebral Ischemia Reduces Infarct Size through Enhanced Collateral Circulation in Murine Focal Cerebral Ischemia.J Stroke Cerebrovasc Dis. 2018 Apr;27(4):831-838. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.068. Epub 2018 Feb 1. J Stroke Cerebrovasc Dis. 2018. PMID: 29395650
-
Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia.Circulation. 2016 Aug 2;134(5):405-21. doi: 10.1161/CIRCULATIONAHA.116.021311. Circulation. 2016. PMID: 27482003 Free PMC article.
-
Remote ischemic conditioning for stroke: A critical systematic review.Int J Stroke. 2024 Mar;19(3):271-279. doi: 10.1177/17474930231191082. Epub 2023 Aug 1. Int J Stroke. 2024. PMID: 37466245 Free PMC article.
-
Remote ischemic conditioning for stroke: clinical data, challenges, and future directions.Ann Clin Transl Neurol. 2018 Nov 15;6(1):186-196. doi: 10.1002/acn3.691. eCollection 2019 Jan. Ann Clin Transl Neurol. 2018. PMID: 30656197 Free PMC article. Review.
Cited by
-
Age affects the association of red blood cell indices with efficacy of remote ischemic conditioning in patients with acute moderate ischemic stroke.Sci Rep. 2024 Sep 29;14(1):22561. doi: 10.1038/s41598-024-74293-9. Sci Rep. 2024. PMID: 39343777 Free PMC article.
-
Potential for in vivo visualization of intracellular pH gradient in the brain using PET imaging.Brain Commun. 2024 May 22;6(3):fcae172. doi: 10.1093/braincomms/fcae172. eCollection 2024. Brain Commun. 2024. PMID: 38863573 Free PMC article.
-
The potential mechanism and clinical application value of remote ischemic conditioning in stroke.Neural Regen Res. 2025 Jun 1;20(6):1613-1627. doi: 10.4103/NRR.NRR-D-23-01800. Epub 2024 Jun 3. Neural Regen Res. 2025. PMID: 38845225 Free PMC article.
-
Inhibition of the JAK2/STAT3 pathway and cell cycle re-entry contribute to the protective effect of remote ischemic pre-conditioning of rat hindlimbs on cerebral ischemia/reperfusion injury.CNS Neurosci Ther. 2023 Mar;29(3):866-877. doi: 10.1111/cns.14023. Epub 2022 Nov 23. CNS Neurosci Ther. 2023. PMID: 36419252 Free PMC article.
-
A review of remote ischemic conditioning as a potential strategy for neural repair poststroke.CNS Neurosci Ther. 2023 Feb;29(2):516-524. doi: 10.1111/cns.14064. Epub 2022 Dec 22. CNS Neurosci Ther. 2023. PMID: 36550592 Free PMC article. Review.
References
-
- Hess DC, Blauenfeldt RA, Andersen G, et al.. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol 2015; 11: 698–710. - PubMed
-
- Landman TRJ, Schoon Y, Warle MC, et al.. Remote ischemic conditioning as an additional treatment for acute ischemic stroke. Stroke 2019; 50: 1934–1939. - PubMed
-
- Hougaard KD, Hjort N, Zeidler D, et al.. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 2014; 45: 159–167. - PubMed
-
- Heusch G.Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 2015; 116: 674–699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

