Estrogen suppresses SOX9 and activates markers of female development in a human testis-derived cell line
- PMID: 32933467
- PMCID: PMC7493336
- DOI: 10.1186/s12860-020-00307-9
Estrogen suppresses SOX9 and activates markers of female development in a human testis-derived cell line
Abstract
Background: The increasing incidence of reproductive disorders in humans has been attributed to in utero exposure to estrogenic endocrine disruptors. In particular, exposure of the developing testis to exogenous estrogen can negatively impact male reproductive health. To determine how estrogens impact human gonad function, we treated the human testis-derived cell line NT2/D1 with estrogen and examined its impact on SOX9 and the expression of key markers of granulosa (ovarian) and Sertoli (testicular) cell development.
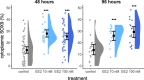
Results: Estrogen successfully activated its cognate receptor (estrogen receptor alpha; ESR1) in NT2/D1 cells. We observed a significant increase in cytoplasmic SOX9 following estrogen treatment. After 48 h of estrogen exposure, mRNA levels of the key Sertoli cell genes SOX9, SRY, AMH, FGF9 and PTGDS were significantly reduced. This was followed by a significant increase in mRNA levels for the key granulosa cell genes FOXL2 and WNT4 after 96 h of estrogen exposure.
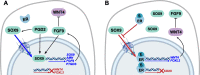
Conclusions: These results are consistent with estrogen's effects on marsupial gonads and show that estrogen has a highly conserved impact on gonadal cell fate decisions that has existed in mammals for over 160 million years. This effect of estrogen presents as a potential mechanism contributing to the significant decrease in male fertility and reproductive health reported over recent decades. Given our widespread exposure to estrogenic endocrine disruptors, their effects on SOX9 and Sertoli cell determination could have considerable impact on the adult testis.
Keywords: Estrogen; Sertoli cells; Sex determination; Testis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Exogenous Oestrogen Impacts Cell Fate Decision in the Developing Gonads: A Potential Cause of Declining Human Reproductive Health.Int J Mol Sci. 2020 Nov 8;21(21):8377. doi: 10.3390/ijms21218377. Int J Mol Sci. 2020. PMID: 33171657 Free PMC article. Review.
-
Oestrogen blocks the nuclear entry of SOX9 in the developing gonad of a marsupial mammal.BMC Biol. 2010 Aug 31;8:113. doi: 10.1186/1741-7007-8-113. BMC Biol. 2010. PMID: 20807406 Free PMC article.
-
DMRT1 prevents female reprogramming in the postnatal mammalian testis.Nature. 2011 Jul 20;476(7358):101-4. doi: 10.1038/nature10239. Nature. 2011. PMID: 21775990 Free PMC article.
-
Mouse Gonad Development in the Absence of the Pro-Ovary Factor WNT4 and the Pro-Testis Factor SOX9.Cells. 2020 Apr 29;9(5):1103. doi: 10.3390/cells9051103. Cells. 2020. PMID: 32365547 Free PMC article.
-
A role for estrogen in somatic cell fate of the mammalian gonad.Chromosome Res. 2012 Jan;20(1):239-45. doi: 10.1007/s10577-011-9260-1. Chromosome Res. 2012. PMID: 22161125 Review.
Cited by
-
Exogenous Oestrogen Impacts Cell Fate Decision in the Developing Gonads: A Potential Cause of Declining Human Reproductive Health.Int J Mol Sci. 2020 Nov 8;21(21):8377. doi: 10.3390/ijms21218377. Int J Mol Sci. 2020. PMID: 33171657 Free PMC article. Review.
-
Assessment of Zearalenone-Induced Cell Survival and of Global Gene Regulation in Mouse TM4 Sertoli Cells.Toxins (Basel). 2022 Jan 26;14(2):98. doi: 10.3390/toxins14020098. Toxins (Basel). 2022. PMID: 35202126 Free PMC article.
-
Identification of Estrogen-Responsive Proteins in Mouse Seminal Vesicles Through Mass Spectrometry-Based Proteomics.Pharmaceuticals (Basel). 2024 Nov 9;17(11):1508. doi: 10.3390/ph17111508. Pharmaceuticals (Basel). 2024. PMID: 39598420 Free PMC article.
-
Impact of Estrogens Present in Environment on Health and Welfare of Animals.Animals (Basel). 2021 Jul 20;11(7):2152. doi: 10.3390/ani11072152. Animals (Basel). 2021. PMID: 34359280 Free PMC article. Review.
-
Vertebrate endocrine disruptors induce sex-reversal in blue mussels.Sci Rep. 2024 Oct 12;14(1):23890. doi: 10.1038/s41598-024-74212-y. Sci Rep. 2024. PMID: 39396059 Free PMC article.
References
-
- Lee PA, Nordenström A, Houk CP, Ahmed SF, Auchus R, Baratz A, et al. Global disorders of sex development update since 2006: perceptions, approach and care. Horm Res Paediatr. 2016;85:158–180. - PubMed
-
- Kalfa N, Paris F, Philibert P, Orsini M, Broussous S, Fauconnet-Servant N, et al. Is hypospadias associated with prenatal exposure to endocrine disruptors? A French collaborative controlled study of a cohort of 300 consecutive children without genetic defect. Eur Urol. 2015;68:1023–1030. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

