Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis
- PMID: 32934000
- PMCID: PMC7493072
- DOI: 10.1136/bmjebm-2020-111536
Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis
Abstract
Objective: To evaluate association between biomarkers and outcomes in COVID-19 hospitalised patients. COVID-19 pandemic has been a challenge. Biomarkers have always played an important role in clinical decision making in various infectious diseases. It is crucial to assess the role of biomarkers in evaluating severity of disease and appropriate allocation of resources.
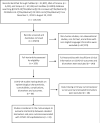
Design and setting: Systematic review and meta-analysis. English full text observational studies describing the laboratory findings and outcomes of COVID-19 hospitalised patients were identified searching PubMed, Web of Science, Scopus, medRxiv using Medical Subject Headings (MeSH) terms COVID-19 OR coronavirus OR SARS-CoV-2 OR 2019-nCoV from 1 December 2019 to 15 August 2020 following Meta-analyses Of Observational Studies in Epidemiology (MOOSE) guidelines.
Participants: Studies having biomarkers, including lymphocyte, platelets, D-dimer, lactate dehydrogenase (LDH), C reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, procalcitonin (PCT) and creatine kinase (CK), and describing outcomes were selected with the consensus of three independent reviewers.
Main outcome measures: Composite poor outcomes include intensive care unit admission, oxygen saturation <90%, invasive mechanical ventilation utilisation, severe disease, in-hospital admission and mortality. The OR and 95% CI were obtained and forest plots were created using random-effects models. Publication bias and heterogeneity were assessed by sensitivity analysis.
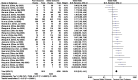
Results: 32 studies with 10 491 confirmed COVID-19 patients were included. We found that lymphopenia (pooled-OR: 3.33 (95% CI: 2.51-4.41); p<0.00001), thrombocytopenia (2.36 (1.64-3.40); p<0.00001), elevated D-dimer (3.39 (2.66-4.33); p<0.00001), elevated CRP (4.37 (3.37-5.68); p<0.00001), elevated PCT (6.33 (4.24-9.45); p<0.00001), elevated CK (2.42 (1.35-4.32); p=0.003), elevated AST (2.75 (2.30-3.29); p<0.00001), elevated ALT (1.71 (1.32-2.20); p<0.00001), elevated creatinine (2.84 (1.80-4.46); p<0.00001) and LDH (5.48 (3.89-7.71); p<0.00001) were independently associated with higher risk of poor outcomes.
Conclusion: Our study found a significant association between lymphopenia, thrombocytopenia and elevated levels of CRP, PCT, LDH, D-dimer and COVID-19 severity. The results have the potential to be used as an early biomarker to improve the management of COVID-19 patients, by identification of high-risk patients and appropriate allocation of healthcare resources in the pandemic.
Keywords: critical care; evidence-based practice; global health; infectious disease medicine; prognosis.
© Author(s) (or their employer(s)) 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures









References
-
- WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020: World Health Organization; 2020. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re... [Accessed 9 Apr 2020].
-
- COVID-19 coronavirus pandemic: Worldometer, 2020. Available: https://www.worldometers.info/coronavirus/#countries [Accessed 20 Aug 2020].
-
- Cases in the U.S.: centers for disease control and prevention, 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html [Accessed 20 Aug 2020].
-
- Who coronavirus disease (COVID-19) Dashboard: World Health organization, 2020. Available: https://covid19.who.int/ [Accessed 20 Aug 2020].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
