Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells
- PMID: 32934215
- PMCID: PMC7492405
- DOI: 10.1038/s41419-020-02956-2
Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells
Erratum in
-
Correction: Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells.Cell Death Dis. 2020 Oct 30;11(10):935. doi: 10.1038/s41419-020-03128-y. Cell Death Dis. 2020. PMID: 33127877 Free PMC article.
Abstract
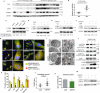
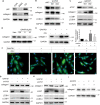
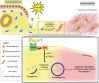
Autophagy can be dynamically induced in response to stresses and is an essential, ubiquitous intracellular recycling system that impacts the fate of damaged resident cells, thereby influencing wound healing. Endometrial fibrosis is a form of abnormal wound healing that causes intrauterine adhesion (IUA) and infertility. We previously demonstrated that overactivated sonic hedgehog (SHH) signaling exacerbated endometrial fibrosis, but the role of autophagy in this process is still unknown. Here, we report that impaired autophagy participates in SHH pathway-induced endometrial fibrosis. Endometrial stroma-myofibroblast transition accompanied by autophagy dysfunction was present in both endometrial biopsies of IUA patients and Amhr2cre/+ R26-SmoM2+/- (AM2) transgenic mouse. Mechanistically, SHH pathway negatively regulated autophagy through pAKT-mTORC1 in a human endometrial stromal cell line (T-HESCs). Furthermore, SHH pathway-mediated fibrosis was partly counteracted by autophagy modulation in both T-HESCs and the murine IUA model. Specifically, the impact of SHH pathway inhibition (GANT61) was reversed by the pharmacological autophagy inhibitor chloroquine (CQ) or RNA interference of autophagy-related gene ATG5 or ATG7. Similar results were obtained from the murine IUA model treated with GANT61 and CQ. Moreover, promoting autophagy with rapamycin reduced fibrosis in the AM2 IUA model to baseline levels. In summary, defective autophagy is involved in SHH pathway-driven endometrial fibrosis, suggesting a potential novel molecular target for IUA treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Reduced expression of SMAD7 and consequent reduction of autophagy promotes endometrial stromal-myofibroblast transition and fibrosis.Mol Hum Reprod. 2024 Oct 12;30(10):gaae036. doi: 10.1093/molehr/gaae036. Mol Hum Reprod. 2024. PMID: 39412480
-
Defective autophagy contributes to endometrial epithelial-mesenchymal transition in intrauterine adhesions.Autophagy. 2022 Oct;18(10):2427-2442. doi: 10.1080/15548627.2022.2038994. Epub 2022 Feb 23. Autophagy. 2022. PMID: 35196191 Free PMC article.
-
Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model.Stem Cell Res Ther. 2019 Aug 14;10(1):257. doi: 10.1186/s13287-019-1368-9. Stem Cell Res Ther. 2019. PMID: 31412924 Free PMC article.
-
Advanced Role of Hippo Signaling in Endometrial Fibrosis: Implications for Intrauterine Adhesion.Chin Med J (Engl). 2017 Nov 20;130(22):2732-2737. doi: 10.4103/0366-6999.218013. Chin Med J (Engl). 2017. PMID: 29133764 Free PMC article. Review.
-
Recent Advances in Understandings Towards Pathogenesis and Treatment for Intrauterine Adhesion and Disruptive Insights from Single-Cell Analysis.Reprod Sci. 2021 Jul;28(7):1812-1826. doi: 10.1007/s43032-020-00343-y. Epub 2020 Oct 30. Reprod Sci. 2021. PMID: 33125685 Free PMC article. Review.
Cited by
-
Integrative analysis links autophagy to intrauterine adhesion and establishes autophagy-related circRNA-miRNA-mRNA regulatory network.Aging (Albany NY). 2023 Aug 23;15(16):8275-8297. doi: 10.18632/aging.204969. Epub 2023 Aug 23. Aging (Albany NY). 2023. PMID: 37616056 Free PMC article.
-
Bone mesenchymal stem cells-derived miR-223-3p-containing exosomes ameliorate lipopolysaccharide-induced acute uterine injury via interacting with endothelial progenitor cells.Bioengineered. 2021 Dec;12(2):10654-10665. doi: 10.1080/21655979.2021.2001185. Bioengineered. 2021. PMID: 34738867 Free PMC article.
-
Application of Bioactive Hydrogels for Functional Treatment of Intrauterine Adhesion.Front Bioeng Biotechnol. 2021 Sep 21;9:760943. doi: 10.3389/fbioe.2021.760943. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34621732 Free PMC article. Review.
-
Photothermal therapy of copper incorporated nanomaterials for biomedicine.Biomater Res. 2023 Nov 24;27(1):121. doi: 10.1186/s40824-023-00461-z. Biomater Res. 2023. PMID: 38001505 Free PMC article. Review.
-
Efficacy of Applying Hyaluronic Acid Gels in the Primary Prevention of Intrauterine Adhesion after Hysteroscopic Myomectomy: A Meta-Analysis of Randomized Controlled Trials.Life (Basel). 2020 Nov 15;10(11):285. doi: 10.3390/life10110285. Life (Basel). 2020. PMID: 33203159 Free PMC article.
References
-
- Evans J, et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 2016;12:654–667. - PubMed
-
- Strowitzki T, Germeyer A, Popovici R, von Wolff M. The human endometrium as a fertility-determining factor. Hum. Reprod. Update. 2006;12:617–630. - PubMed
-
- Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome–one century later. Fertil. Steril. 2008;89:759–779. - PubMed
-
- Deans R, Abbott J. Review of intrauterine adhesions. J. Minim. Invasive Gynecol. 2010;17:555–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

