NEMF mutations that impair ribosome-associated quality control are associated with neuromuscular disease
- PMID: 32934225
- PMCID: PMC7494853
- DOI: 10.1038/s41467-020-18327-6
NEMF mutations that impair ribosome-associated quality control are associated with neuromuscular disease
Erratum in
-
Author Correction: NEMF mutations that impair ribosome-associated quality control are associated with neuromuscular disease.Nat Commun. 2020 Oct 1;11(1):5022. doi: 10.1038/s41467-020-18941-4. Nat Commun. 2020. PMID: 33004807 Free PMC article.
Abstract
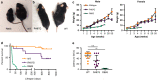
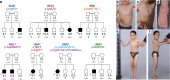
A hallmark of neurodegeneration is defective protein quality control. The E3 ligase Listerin (LTN1/Ltn1) acts in a specialized protein quality control pathway-Ribosome-associated Quality Control (RQC)-by mediating proteolytic targeting of incomplete polypeptides produced by ribosome stalling, and Ltn1 mutation leads to neurodegeneration in mice. Whether neurodegeneration results from defective RQC and whether defective RQC contributes to human disease have remained unknown. Here we show that three independently-generated mouse models with mutations in a different component of the RQC complex, NEMF/Rqc2, develop progressive motor neuron degeneration. Equivalent mutations in yeast Rqc2 selectively interfere with its ability to modify aberrant translation products with C-terminal tails which assist with RQC-mediated protein degradation, suggesting a pathomechanism. Finally, we identify NEMF mutations expected to interfere with function in patients from seven families presenting juvenile neuromuscular disease. These uncover NEMF's role in translational homeostasis in the nervous system and implicate RQC dysfunction in causing neurodegeneration.
Conflict of interest statement
J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals, Novartis, and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from genetic testing offered at Baylor Genetics (
Figures



References
-
- Defenouillere Q, Fromont-Racine M. The ribosome-bound quality control complex: from aberrant peptide clearance to proteostasis maintenance. Curr. Genet. 2017;63:997–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

