Pharmacophore hybridisation and nanoscale assembly to discover self-delivering lysosomotropic new-chemical entities for cancer therapy
- PMID: 32934241
- PMCID: PMC7493904
- DOI: 10.1038/s41467-020-18399-4
Pharmacophore hybridisation and nanoscale assembly to discover self-delivering lysosomotropic new-chemical entities for cancer therapy
Erratum in
-
Author Correction: Pharmacophore hybridisation and nanoscale assembly to discover self-delivering lysosomotropic new-chemical entities for cancer therapy.Nat Commun. 2021 Mar 25;12(1):2013. doi: 10.1038/s41467-021-22419-2. Nat Commun. 2021. PMID: 33767181 Free PMC article. No abstract available.
Abstract
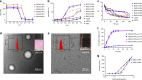
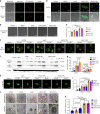
Integration of the unique advantages of the fields of drug discovery and drug delivery is invaluable for the advancement of drug development. Here we propose a self-delivering one-component new-chemical-entity nanomedicine (ONN) strategy to improve cancer therapy through incorporation of the self-assembly principle into drug design. A lysosomotropic detergent (MSDH) and an autophagy inhibitor (Lys05) are hybridised to develop bisaminoquinoline derivatives that can intrinsically form nanoassemblies. The selected BAQ12 and BAQ13 ONNs are highly effective in inducing lysosomal disruption, lysosomal dysfunction and autophagy blockade and exhibit 30-fold higher antiproliferative activity than hydroxychloroquine used in clinical trials. These single-drug nanoparticles demonstrate excellent pharmacokinetic and toxicological profiles and dramatic antitumour efficacy in vivo. In addition, they are able to encapsulate and deliver additional drugs to tumour sites and are thus promising agents for autophagy inhibition-based combination therapy. Given their transdisciplinary advantages, these BAQ ONNs have enormous potential to improve cancer therapy.
Conflict of interest statement
Y.L. and Z.M. are the co-inventors on a pending patent application on the hybrid compounds and the resulting nanoformulations. The remaining authors declare no competing interests.
Figures




Similar articles
-
Lys05: a new lysosomal autophagy inhibitor.Autophagy. 2012 Sep;8(9):1383-4. doi: 10.4161/auto.20958. Epub 2012 Aug 10. Autophagy. 2012. PMID: 22878685 Free PMC article.
-
Nanomedicine: An effective tool in cancer therapy.Int J Pharm. 2018 Apr 5;540(1-2):132-149. doi: 10.1016/j.ijpharm.2018.02.007. Epub 2018 Feb 7. Int J Pharm. 2018. PMID: 29427746 Review.
-
Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency.Proc Natl Acad Sci U S A. 2012 May 22;109(21):8253-8. doi: 10.1073/pnas.1118193109. Epub 2012 May 7. Proc Natl Acad Sci U S A. 2012. PMID: 22566612 Free PMC article.
-
Nanoscale drug delivery for targeted chemotherapy.Cancer Lett. 2016 Aug 28;379(1):24-31. doi: 10.1016/j.canlet.2016.05.023. Epub 2016 May 25. Cancer Lett. 2016. PMID: 27235607 Review.
-
Nanomedicine in cancer treatment.Nanomedicine. 2005 Jun;1(2):191-2. doi: 10.1016/j.nano.2005.04.001. Nanomedicine. 2005. PMID: 17292078 Review. No abstract available.
Cited by
-
Transformable nanoparticles to bypass biological barriers in cancer treatment.Nanoscale Adv. 2022 Sep 14;4(21):4470-4480. doi: 10.1039/d2na00485b. eCollection 2022 Oct 25. Nanoscale Adv. 2022. PMID: 36341301 Free PMC article. Review.
-
GSH-responsive poly-resveratrol based nanoparticles for effective drug delivery and reversing multidrug resistance.Drug Deliv. 2022 Dec;29(1):229-237. doi: 10.1080/10717544.2021.2023700. Drug Deliv. 2022. PMID: 35001781 Free PMC article.
-
Autophagy and the Lysosomal System in Cancer.Cells. 2021 Oct 14;10(10):2752. doi: 10.3390/cells10102752. Cells. 2021. PMID: 34685734 Free PMC article. Review.
-
Genetic assessment of pathogenic germline alterations in lysosomal genes among Asian patients with pancreatic ductal adenocarcinoma.J Transl Med. 2023 Oct 17;21(1):730. doi: 10.1186/s12967-023-04549-x. J Transl Med. 2023. PMID: 37848935 Free PMC article.
-
Structure-activity relationship of pharmacophores and toxicophores: the need for clinical strategy.Daru. 2024 Dec;32(2):781-800. doi: 10.1007/s40199-024-00525-y. Epub 2024 Jun 27. Daru. 2024. PMID: 38935265 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

