Defining diagnostic cutoffs in neurological patients for serum very long chain fatty acids (VLCFA) in genetically confirmed X-Adrenoleukodystrophy
- PMID: 32934269
- PMCID: PMC7494896
- DOI: 10.1038/s41598-020-71248-8
Defining diagnostic cutoffs in neurological patients for serum very long chain fatty acids (VLCFA) in genetically confirmed X-Adrenoleukodystrophy
Abstract
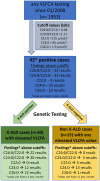
X-linked Adrenoleukodystrophy (X-ALD) is caused by mutations in the ABCD1 gene resulting in the accumulation of very long chain fatty acids (VLCFA). X-ALD is the most common peroxisomal disorder with adult patients (male and female) presenting with progressive spastic paraparesis with bladder disturbance, sensory ataxia with impaired vibration sense, and leg pain. 80% of male X-ALD patients have an adrenal failure, while adrenal dysfunction is rare in women with X-ALD. The objective of this study was to define optimal serum VLCFA cutoff values in patients with X-ALD-like phenotypes for the differentiation of genetically confirmed X-ALD and Non-X-ALD individuals. Three groups were included into this study: a) X-ALD cases with confirmed ABCD1 mutations (n = 34) and two Non-X-ALD cohorts: b) Patients with abnormal serum VCLFA levels despite negative testing for ABCD1 mutations (n = 15) resulting from a total of 1,953 VLCFA tests c) Phenotypically matching patients as Non-X-ALD controls (n = 104). Receiver operating curve analysis was used to optimize VLCFA cutoff values, which differentiate patients with genetically confirmed X-ALD and Non-X-ALD individuals. The serum concentration of C26:0 was superior to C24:0 for the detection of X-ALD. The best differentiation of Non-X-ALD and X-ALD individuals was obtained with a cutoff value of < 1.0 for the C24:0/C22:0 ratio resulting in a sensitivity of 97%, a specificity of 94.1% and a positive predictive value (PPV) of 83.8% for true X-ALD. Our findings further suggested a cutoff of < 0.02 for the ratio C26:0/C22:0 leading to a sensitivity of 90.9%, a specificity of 95.0%, and a PPV of 80.6%. Pearson correlation indicated a significant positive association between total blood cholesterol and VLCFA values. Usage of serum VLCFA are economical and established biomarkers suitable for the guidance of genetic testing matching the X-ALD phenotype. We suggest using our new optimized cutoff values, especially the two ratios (C24:0/C22:0 and C26:0/C22:0), in combination with standard lipid profiles.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Astrocytes and mitochondria from adrenoleukodystrophy protein (ABCD1)-deficient mice reveal that the adrenoleukodystrophy-associated very long-chain fatty acids target several cellular energy-dependent functions.Biochim Biophys Acta. 2015 May;1852(5):925-36. doi: 10.1016/j.bbadis.2015.01.005. Epub 2015 Jan 10. Biochim Biophys Acta. 2015. PMID: 25583114
-
Comparison of C26:0-carnitine and C26:0-lysophosphatidylcholine as diagnostic markers in dried blood spots from newborns and patients with adrenoleukodystrophy.Mol Genet Metab. 2017 Dec;122(4):209-215. doi: 10.1016/j.ymgme.2017.10.012. Epub 2017 Oct 28. Mol Genet Metab. 2017. PMID: 29089175
-
Impaired very long-chain acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction.J Biol Chem. 2013 Jun 28;288(26):19269-79. doi: 10.1074/jbc.M112.445445. Epub 2013 May 13. J Biol Chem. 2013. PMID: 23671276 Free PMC article.
-
[X-linked adrenoleukodystrophy].Ann Endocrinol (Paris). 2007 Dec;68(6):403-11. doi: 10.1016/j.ando.2007.04.002. Epub 2007 May 29. Ann Endocrinol (Paris). 2007. PMID: 17532287 Review. French.
-
Biochemical aspects of X-linked adrenoleukodystrophy.Brain Pathol. 2010 Jul;20(4):831-7. doi: 10.1111/j.1750-3639.2010.00391.x. Brain Pathol. 2010. PMID: 20626744 Free PMC article. Review.
Cited by
-
Diagnosing X-Linked Adrenoleukodystrophy after Implementation of Newborn Screening: A Reference Laboratory Perspective.Int J Neonatal Screen. 2023 Nov 2;9(4):64. doi: 10.3390/ijns9040064. Int J Neonatal Screen. 2023. PMID: 37987477 Free PMC article.
-
Perceptions and Experiences of Families of Infants Diagnosed with X-Linked Adrenoleukodystrophy (X-ALD) via Newborn Screening in Georgia and Kentucky.J Prim Care Community Health. 2025 Jan-Dec;16:21501319251337182. doi: 10.1177/21501319251337182. Epub 2025 Jun 29. J Prim Care Community Health. 2025. PMID: 40581955 Free PMC article.
-
Primary Adrenal Insufficiency in a Boy with Type I Diabetes: The Importance of Considering X-linked Adrenoleukodystrophy.J Endocr Soc. 2022 Apr 12;6(5):bvac039. doi: 10.1210/jendso/bvac039. eCollection 2022 May 1. J Endocr Soc. 2022. PMID: 35450414 Free PMC article.
-
Genetic analysis of the X-linked Adrenoleukodystrophy ABCD1 gene in Drosophila uncovers a role in Peroxisomal dynamics.bioRxiv [Preprint]. 2024 Sep 25:2024.09.23.614586. doi: 10.1101/2024.09.23.614586. bioRxiv. 2024. PMID: 39386423 Free PMC article. Preprint.
-
Peroxisomal ABC Transporters: An Update.Int J Mol Sci. 2021 Jun 5;22(11):6093. doi: 10.3390/ijms22116093. Int J Mol Sci. 2021. PMID: 34198763 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

