Robust rapid-setting antibacterial liquid bandages
- PMID: 32934279
- PMCID: PMC7492242
- DOI: 10.1038/s41598-020-71586-7
Robust rapid-setting antibacterial liquid bandages
Abstract
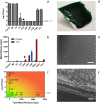
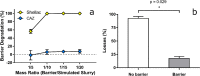
Bandaging is a steadfast but time-consuming component of wound care with limited technical advancements to date. Bandages must be changed and infection risk managed. Rapid-set liquid bandages are efficient alternatives but lack durability or inherent infection control. We show here that antibacterial zinc (Zn) and copper (Cu) species greatly enhance the barrier properties of the natural, waterproof, bio-adhesive polymer, shellac. The material demonstrated marked antibacterial contact properties and, in ex-vivo studies, effectively locked-in pre-applied therapeutics. When challenged in vivo with the polybacterial bovine wound infection 'digital dermatitis', Zn/Cu-shellac adhered rapidly and robustly over pre-applied antibiotic. The bandage self-degraded, appropriately, over 7 days despite extreme conditions (faecal slurry). Treatment was well-tolerated and clinical improvement was observed in animal mobility. This new class of bandage has promise for challenging topical situations in humans and other animals, especially away from controlled, sterile clinical settings where wounds urgently require protection from environmental and bacterial contamination.
Conflict of interest statement
WDT, CAPB, NF and JJP are inventors on a patent application describing the use of antimicrobial liquid bandages and are seeking to spin out the technology via the University of Cambridge. The remaining authors declare no further competing interests.
Figures

References
-
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: current advances and future directions. J. Appl. Polym. Sci. 2019;136:47738. doi: 10.1002/app.47738. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

