Molecular mechanisms underlying AMH elevation in hyperoestrogenic states in males
- PMID: 32934281
- PMCID: PMC7492256
- DOI: 10.1038/s41598-020-71675-7
Molecular mechanisms underlying AMH elevation in hyperoestrogenic states in males
Abstract
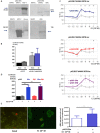
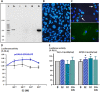
Anti-Müllerian hormone (AMH) is secreted by Sertoli cells of the testes from early fetal life until puberty, when it is downregulated by androgens. In conditions like complete androgen insensitivity syndrome (CAIS), AMH downregulation does not occur and AMH increases at puberty, due in part to follicle-stimulating hormone (FSH) effect. However, other conditions like Peutz-Jeghers syndrome (PJS), characterised by low FSH, also have increased AMH. Because both CAIS and PJS may present as hyperoestrogenic states, we tested the hypothesis that oestradiol (E2) upregulates AMH expression in peripubertal Sertoli cells and explored the molecular mechanisms potentially involved. The results showed that E2 is capable of inducing an upregulation of endogenous AMH and of the AMH promoter activity in the prepubertal Sertoli cell line SMAT1, signalling through ERα binding to a specific ERE sequence present on the hAMH promoter. A modest action was also mediated through the membrane oestrogen receptor GPER. Additionally, the existence of ERα expression in Sertoli cells in patients with CAIS was confirmed by immunohistochemistry. The evidence presented here provides biological plausibility to the hypothesis that testicular AMH production increases in clinical conditions in response to elevated oestrogen levels.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

