Comparison of the effectiveness and safety of travoprost and latanoprost for the management of open-angle glaucoma given as an evening dose
- PMID: 32934689
- PMCID: PMC7472139
- DOI: 10.3892/etm.2020.9152
Comparison of the effectiveness and safety of travoprost and latanoprost for the management of open-angle glaucoma given as an evening dose
Abstract
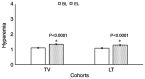
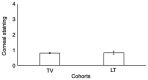
As intraocular pressure (IOP) is primarily higher in the morning, an evening dose of prostaglandin analogs is typically used as monotherapy to decrease IOP in patients with open-angle glaucoma. Travoprost (TV) has reported efficacy in treating open-angle glaucoma; however, the safety and efficacy may be different compared with that for latanoprost (LT). The aim of the present study was to compare the effectiveness and safety of an evening dose of TV compared with that of LT in treating open-angle glaucoma. Data including IOP, results of lid and slit-lamp examination and ophthalmoscopy, as well as adverse effects in 250 affected eyes from patients with open-angle glaucoma who received either TV (n=89) or LT (n=161) once in the evening for 3-months were included in the analyses. At the end of treatment, TV (23.45±1.52 vs. 19.15±1.01 mmHg; P<0.0001) and LT (23.93±2.11 vs. 19.45±1.11 mmHg; P<0.0001) successfully lowered the IOP. In addition, there was no significant difference in the reduction of IOP values at the end of treatment between the two groups (P=0.120). Furthermore, there were no adverse effects on visual acuity (P>0.05), except for non-visual acuity, for example hyperemia (P<0.0001 for both groups), while there was a significant increase in the number of patients with dry eyes receiving TV (P=0.020) and a significant increase with eyelid swelling (P=0.036) and headache (P=0.037) in patients receiving LT. In conclusion, evening doses of TV and LT had the same efficacy and manageable adverse effects in the treatment of open-angle glaucoma (level of evidence, 3).
Keywords: evening dosing; hyperemia; intraocular pressure; latanoprost; open-angle glaucoma; travoprost.
Copyright © 2020, Spandidos Publications.
Figures



Similar articles
-
A 1-year study to compare the efficacy and safety of once-daily travoprost 0.004%/timolol 0.5% to once-daily latanoprost 0.005%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension.Eur J Ophthalmol. 2007 Mar-Apr;17(2):183-90. doi: 10.1177/112067210701700206. Eur J Ophthalmol. 2007. PMID: 17415690 Clinical Trial.
-
A 6-week, double-masked, parallel-group study of the efficacy and safety of travoprost 0.004% compared with latanoprost 0:005%/timolol 0.5% in patients with primary open-angle glaucoma or ocular hypertension.Clin Ther. 2006 Mar;28(3):332-9. doi: 10.1016/j.clinthera.2006.03.001. Clin Ther. 2006. PMID: 16750448 Clinical Trial.
-
Comparison of the diurnal ocular hypotensive efficacy of travoprost and latanoprost over a 44-hour period in patients with elevated intraocular pressure.Clin Ther. 2004 Jan;26(1):84-91. doi: 10.1016/s0149-2918(04)90008-2. Clin Ther. 2004. PMID: 14996520 Clinical Trial.
-
Mixed treatment comparison and meta-regression of the efficacy and safety of prostaglandin analogues and comparators for primary open-angle glaucoma and ocular hypertension.Curr Med Res Opin. 2010 Mar;26(3):511-28. doi: 10.1185/03007990903498786. Curr Med Res Opin. 2010. PMID: 20014995 Review.
-
Latanoprost : an update of its use in glaucoma and ocular hypertension.Drugs Aging. 2003;20(8):597-630. doi: 10.2165/00002512-200320080-00005. Drugs Aging. 2003. PMID: 12795627 Review.
Cited by
-
Recent Progress on Photocatalytic Synthesis of Ester Derivatives and Reaction Mechanisms.Top Curr Chem (Cham). 2021 Oct 19;379(6):42. doi: 10.1007/s41061-021-00355-5. Top Curr Chem (Cham). 2021. PMID: 34668085 Review.
References
-
- Stalmans I, Oddone F, Cordeiro MF, Hommer A, Montesano G, Ribeiro L, Sunaric-Mégevand G, Rossetti L. Comparison of preservative-free latanoprost and preservative-free bimatoprost in a multicenter, randomized, investigator-masked cross-over clinical trial, the SPORT trial. Graefes Arch Clin Exp Ophthalmol. 2016;254:1151–1158. doi: 10.1007/s00417-016-3299-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
