Nuclear receptor FXR impairs SK-Hep-1 cell migration and invasion by inhibiting the Wnt/β-catenin signaling pathway
- PMID: 32934729
- PMCID: PMC7471648
- DOI: 10.3892/ol.2020.12022
Nuclear receptor FXR impairs SK-Hep-1 cell migration and invasion by inhibiting the Wnt/β-catenin signaling pathway
Abstract
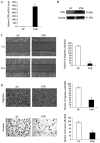
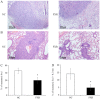
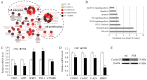
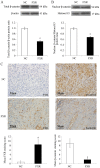
Recently, the nuclear receptor farnesoid X receptor (FXR) has been considered to be a liver tumor suppressor. However, the role of FXR in liver cancer invasion and metastasis remains unclear. The results of the current study demonstrated that FXR suppressed the migratory and invasive capacities of SK-Hep-1 cells in vitro and that FXR overexpression inhibited local invasion and lung metastasis of SK-Hep-1 ×enografts in vivo. Bioinformatics analysis of the gene expression profile of SK-Hep-1 cells with different FXR levels indicated that FXR may regulate the Wnt/β-catenin pathway. Compared with controls, FXR-overexpressing SK-Hep-1 cells exhibited decreased expression of β-catenin target genes and reduced nuclear translocation of β-catenin proteins in vitro and in vivo. In conclusion, these results indicated that FXR may suppress SK-Hep-1 cell invasion and metastasis by suppressing the Wnt/β-catenin signaling pathway. The current study provided novel insight into the diagnosis and treatment of liver cancer.
Keywords: FXR; SK-Hep-1 cell; Wnt/β-catenin; invasion and metastasis.
Copyright: © Li et al.
Figures
Similar articles
-
Metallothionein 1H (MT1H) functions as a tumor suppressor in hepatocellular carcinoma through regulating Wnt/β-catenin signaling pathway.BMC Cancer. 2017 Feb 28;17(1):161. doi: 10.1186/s12885-017-3139-2. BMC Cancer. 2017. PMID: 28241806 Free PMC article.
-
Effects and mechanism of the bile acid (farnesoid X) receptor on the Wnt/β-catenin signaling pathway in colon cancer.Oncol Lett. 2020 Jul;20(1):337-345. doi: 10.3892/ol.2020.11545. Epub 2020 Apr 16. Oncol Lett. 2020. PMID: 32565960 Free PMC article.
-
Farnesoid X receptor associates with β-catenin and inhibits its activity in hepatocellular carcinoma.Oncotarget. 2015 Feb 28;6(6):4226-38. doi: 10.18632/oncotarget.2899. Oncotarget. 2015. PMID: 25650661 Free PMC article.
-
FXR blocks the growth of liver cancer cells through inhibiting mTOR-s6K pathway.Biochem Biophys Res Commun. 2016 May 27;474(2):351-356. doi: 10.1016/j.bbrc.2016.04.106. Epub 2016 Apr 22. Biochem Biophys Res Commun. 2016. PMID: 27109477
-
Targeting Farnesoid X receptor (FXR) for developing novel therapeutics against cancer.Mol Biomed. 2021 Jul 10;2(1):21. doi: 10.1186/s43556-021-00035-2. Mol Biomed. 2021. PMID: 35006466 Free PMC article. Review.
Cited by
-
FHL1: A novel diagnostic marker for papillary thyroid carcinoma.Pathol Int. 2024 Sep;74(9):520-529. doi: 10.1111/pin.13467. Epub 2024 Aug 9. Pathol Int. 2024. PMID: 39119938 Free PMC article.
-
Enhancement of Farnesoid X Receptor Inhibits Migration, Adhesion and Angiogenesis through Proteasome Degradation and VEGF Reduction in Bladder Cancers.Int J Mol Sci. 2022 May 9;23(9):5259. doi: 10.3390/ijms23095259. Int J Mol Sci. 2022. PMID: 35563650 Free PMC article.
-
Pleiotropic roles of FXR in liver and colorectal cancers.Mol Cell Endocrinol. 2022 Mar 1;543:111543. doi: 10.1016/j.mce.2021.111543. Epub 2022 Jan 4. Mol Cell Endocrinol. 2022. PMID: 34995680 Free PMC article.
References
LinkOut - more resources
Full Text Sources
