Endothelial cells on the move: dynamics in vascular morphogenesis and disease
- PMID: 32935077
- PMCID: PMC7487603
- DOI: 10.1530/VB-20-0007
Endothelial cells on the move: dynamics in vascular morphogenesis and disease
Abstract
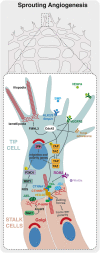
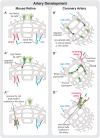
The vascular system is a hierarchically organized network of blood vessels that play crucial roles in embryogenesis, homeostasis and disease. Blood vessels are built by endothelial cells - the cells lining the interior of blood vessels - through a process named vascular morphogenesis. Endothelial cells react to different biomechanical signals in their environment by adjusting their behavior to: (1) invade, proliferate and fuse to form new vessels (angiogenesis); (2) remodel, regress and establish a hierarchy in the network (patterning); and (3) maintain network stability (quiescence). Each step involves the coordination of endothelial cell differentiation, proliferation, polarity, migration, rearrangements and shape changes to ensure network integrity and an efficient barrier between blood and tissues. In this review, we highlighted the relevance and the mechanisms involving endothelial cell migration during different steps of vascular morphogenesis. We further present evidence on how impaired endothelial cell dynamics can contribute to pathology.
Keywords: angiogenesis; cell migration; cell polarity; vascular disease; vascular remodelling.
© 2020 The authors.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

